Question: . 3. Define formal Charge, Bond Angle & Lone pairs: Formal Charge - Valence electrons - (bonds + dots) Indicate the bond angles, lone pairs
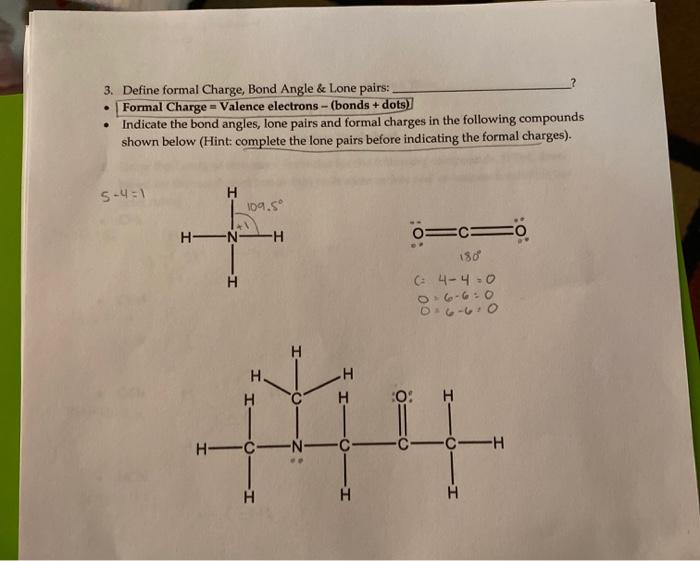
. 3. Define formal Charge, Bond Angle & Lone pairs: Formal Charge - Valence electrons - (bonds + dots) Indicate the bond angles, lone pairs and formal charges in the following compounds shown below (Hint: complete the lone pairs before indicating the formal charges). 5.4=1 H 109.5 HN -H O=CE =0 180 (4-40 06-6:0 3.6.6.0 H H H H H 0 H -N- C C -CH H H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


