Question: Redraw structures and add electrons and formal charge Hide image transcript Resonance and isomers Activity Follow the directions below for each of the following structures
Redraw structures and add electrons and formal charge
Hide image transcript
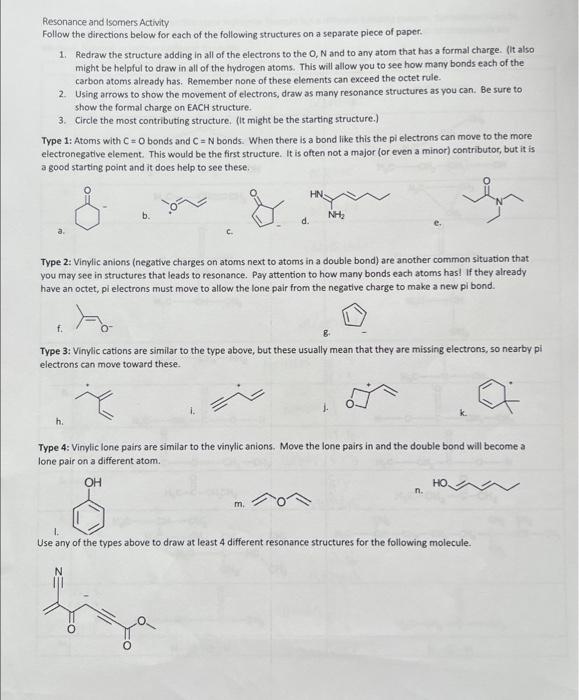
Resonance and isomers Activity Follow the directions below for each of the following structures on a separate piece of paper. Redraw the structure adding in all of the electrons to the ON and to any atom that has a formal charge. It also might be helpful to draw in all of the hydrogen atoms. This will allow you to see how many bonds each of the carbon atoms already has. Remember none of these elements can exceed the octet rule. Using arrows to show the movement of electrons, draw as many resonance structures as you can. Be sure to show the formal charge on EACH structure. Circle the most contributing structure, it might be the starting structure. Type : Atoms with CO bonds and CN bonds. When there is a bond like this the pi electrons can move to the more electronegative element. This would be the first structure. It is often not a major or even a minor contributor, but it is a good starting point and it does help to see these. a b c d e Type : Vinylic anions negative charges on atoms next to atoms in a double bond are another common situation that you may see in structures that leads to resonance. Pay attention to how many bonds each atoms has! if they already have an octet, pl electrons must move to allow the lone pair from the negative charge to make a new pi bond. B Type : Vinylic cations are similar to the type above, but these usually mean that they are missing electrons, so nearby pi electrons can move toward these. h k Type : Vinylic lone pairs are similar to the vinylic anions. Move the lone pairs in and the double bond will become a lone pair on a different atom. m Use any of the types above to draw at least different resonance structures for the following molecule
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


