Question: 3- For a mixture of component A and component B, (a) Determine the partial molar enthalpy of component A and component B at 50mol. %A
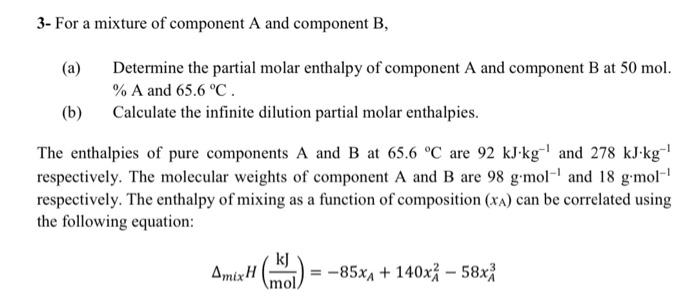
3- For a mixture of component A and component B, (a) Determine the partial molar enthalpy of component A and component B at 50mol. %A and 65.6C. (b) Calculate the infinite dilution partial molar enthalpies. The enthalpies of pure components A and B at 65.6C are 92kJkg1 and 278kJkg1 respectively. The molecular weights of component A and B are 98gmol1 and 18gmol1 respectively. The enthalpy of mixing as a function of composition (xA) can be correlated using the following equation: mixH(molkJ)=85xA+140xA258xA3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


