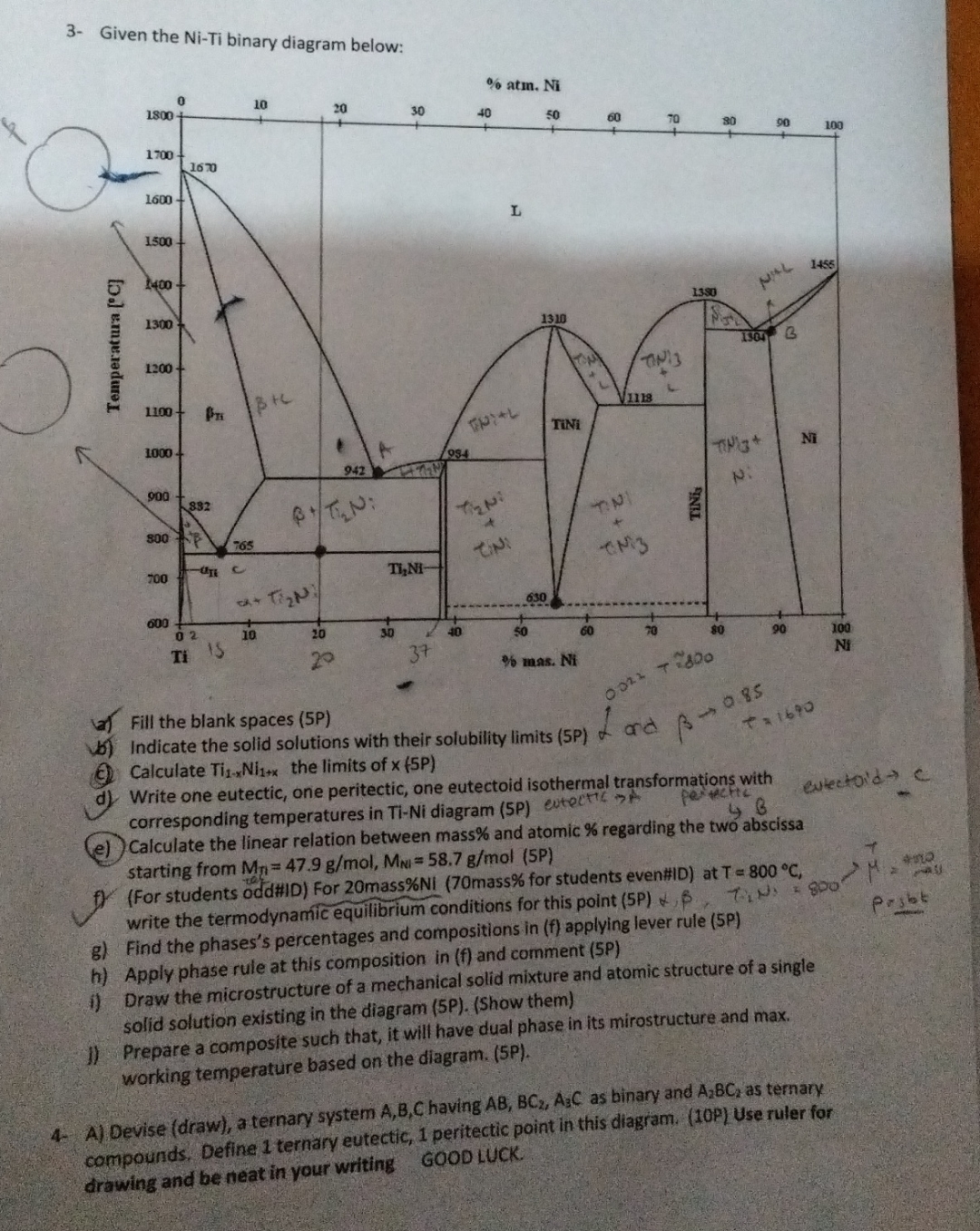
Question: 3 - Given the Ni - Ti binary diagram below: % atm. N i a ) Fill the blank spaces ( 5 P ) b
Given the NiTi binary diagram below:
atm.
a Fill the blank spaces P
b Indicate the solid solutions with their solubility limits P
c Calculate the limits of
d Write one eutectic, one peritectic, one eutectoid isothermal transformations with corresponding temperatures in TiNi diagram P evtect pe CctiC
ewlectoid
e Calculate the linear relation between mass and atomic regarding the two abscissa starting from
fFor students odd#ID For mass mass for students even#ID at write the termodynamic equilibrium conditions for this point Poro tomo casis
g Find the phases's percentages and compositions in applying lever rule P
h Apply phase rule at this composition in f and comment P
i Draw the microstructure of a mechanical solid mixture and atomic structure of a single solid solution existing in the diagram PShow them
Prepare a composite such that, it will have dual phase in its mirostructure and max. working temperature based on the diagram. P
A Devise draw a ternary system having as binary and as ternary compounds. Define ternary eutectic, peritectic point in this diagram. P Use ruler for drawing and be neat in your writing GOOD LUCK.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


