Question: (3) Let's consider we have a reaction A B + C, taking place in a constant- volume batch reactor. The concentration of A has been
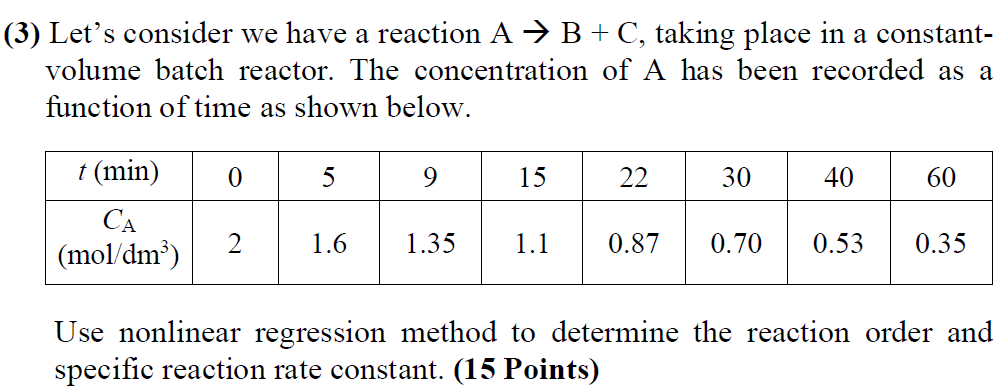
(3) Let's consider we have a reaction A B + C, taking place in a constant- volume batch reactor. The concentration of A has been recorded as a function of time as shown below. 0 5 9 15 22 30 40 60 t(min) (mol/dm) 2 1.6 1.35 1.1 0.87 0.70 0.53 0.35 Use nonlinear regression method to determine the reaction order and specific reaction rate constant. (15 Points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


