Question: 3. The stepwise stability constant, K at 25 C in an aqueous solution for the formation of copper and nickel complexes are shown below. [M(en)3]3+
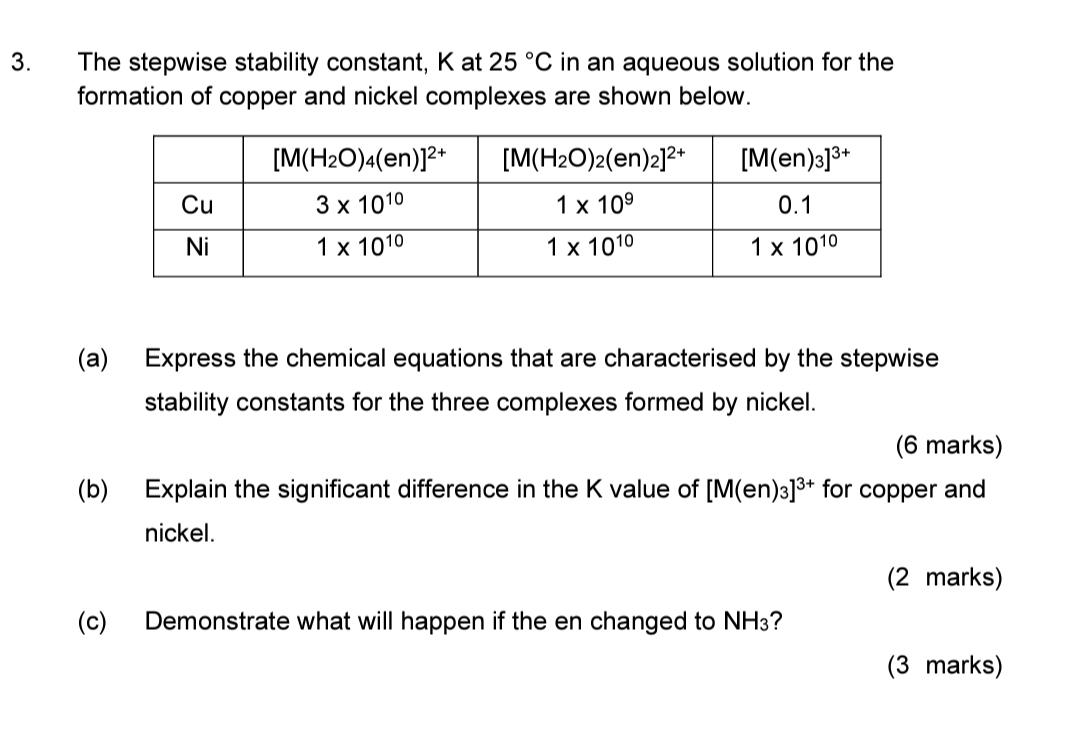
3. The stepwise stability constant, K at 25 C in an aqueous solution for the formation of copper and nickel complexes are shown below. [M(en)3]3+ [M(H2O)4(en)]2+ 3 x 1010 1 x 1010 [M(H2O)2(en)2]2+ 1 x 109 Cu 0.1 Ni 1 x 1010 1 x 1010 (a) Express the chemical equations that are characterised by the stepwise stability constants for the three complexes formed by nickel. (6 marks) Explain the significant difference in the K value of [M(en)3]3+ for copper and nickel. (b) (2 marks) (c) Demonstrate what will happen if the en changed to NH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


