Question: 3. Through using a Clausius-Clapeyron plot based off measured data for a given solution, you generate a line of best fit with the following equation:
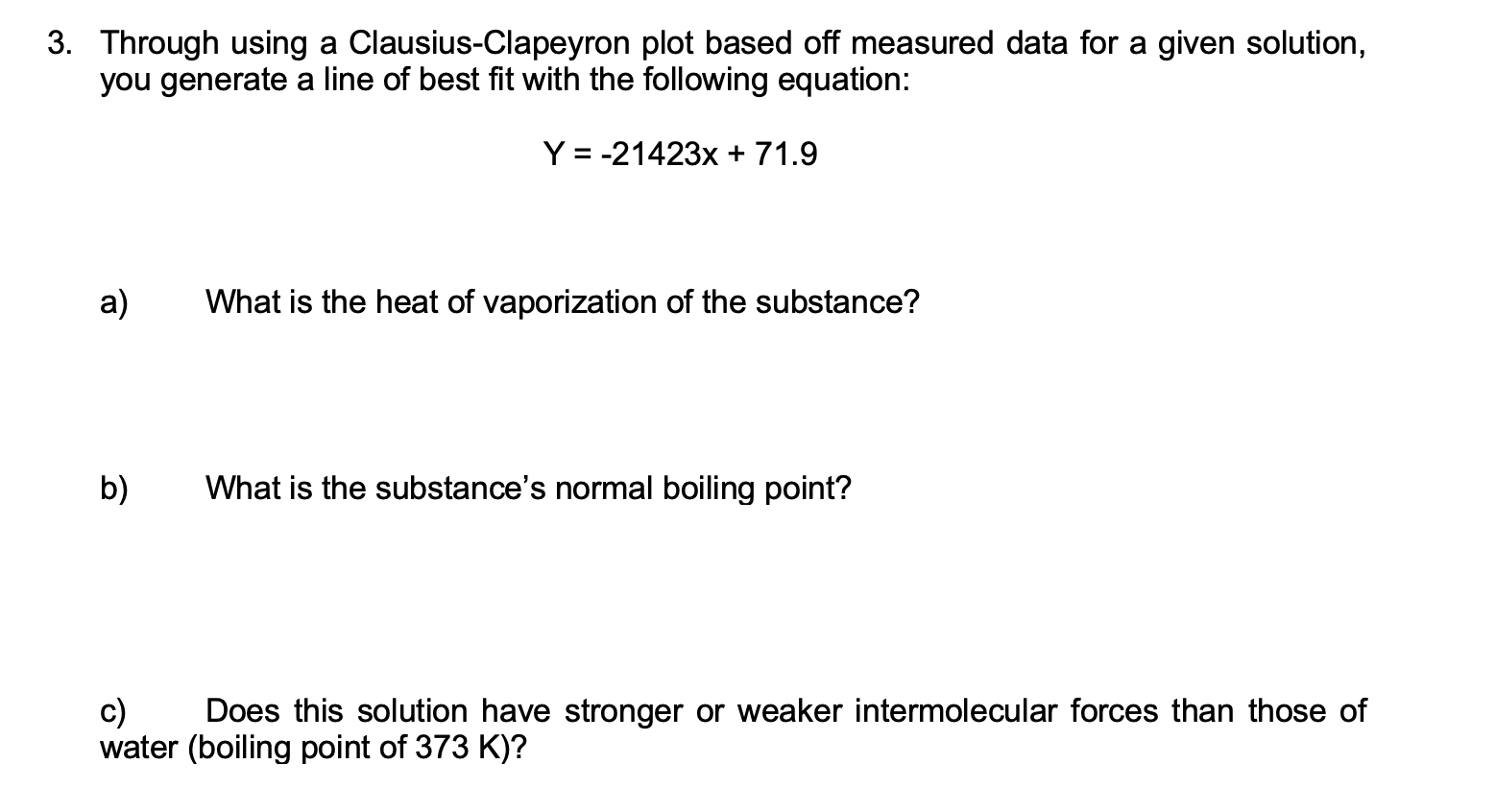
3. Through using a Clausius-Clapeyron plot based off measured data for a given solution, you generate a line of best fit with the following equation: Y = -21423x + 71.9 a) What is the heat of vaporization of the substance? b) What is the substance's normal boiling point? c) Does this solution have stronger or weaker intermolecular forces than those of water (boiling point of 373 K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


