Question: 3. Which does the following graph represent, a cyclohexane-toluene mixture or an ethanol-water mixture? Does it represent a simple distillation or a fractional distillation? Explain
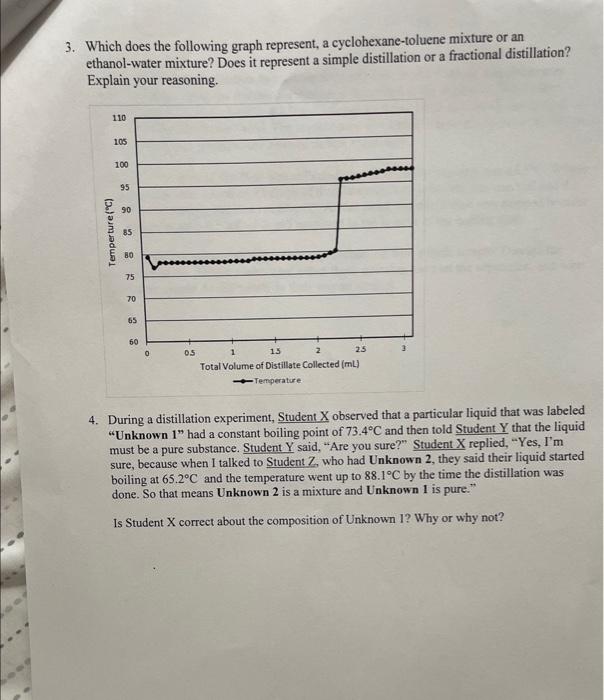
3. Which does the following graph represent, a cyclohexane-toluene mixture or an ethanol-water mixture? Does it represent a simple distillation or a fractional distillation? Explain your reasoning. 4. During a distillation experiment, Student X observed that a particular liquid that was labeled "Unknown 1 " had a constant boiling point of 73.4C and then told Student Y that the liquid must be a pure substance. Student Y said, "Are you sure?" Student X replied, "Yes, I'm sure, because when I talked to Student Z, who had Unknown 2, they said their liquid started boiling at 65.2C and the temperature went up to 88.1C by the time the distillation was done. So that means Unknown 2 is a mixture and Unknown 1 is pure." Is Student X correct about the composition of Unknown 1? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


