Question: 3. Write down how you got the formula for the precipitate that forms when a solution of Ba(OH)2 is added to a solution of H2SO4.
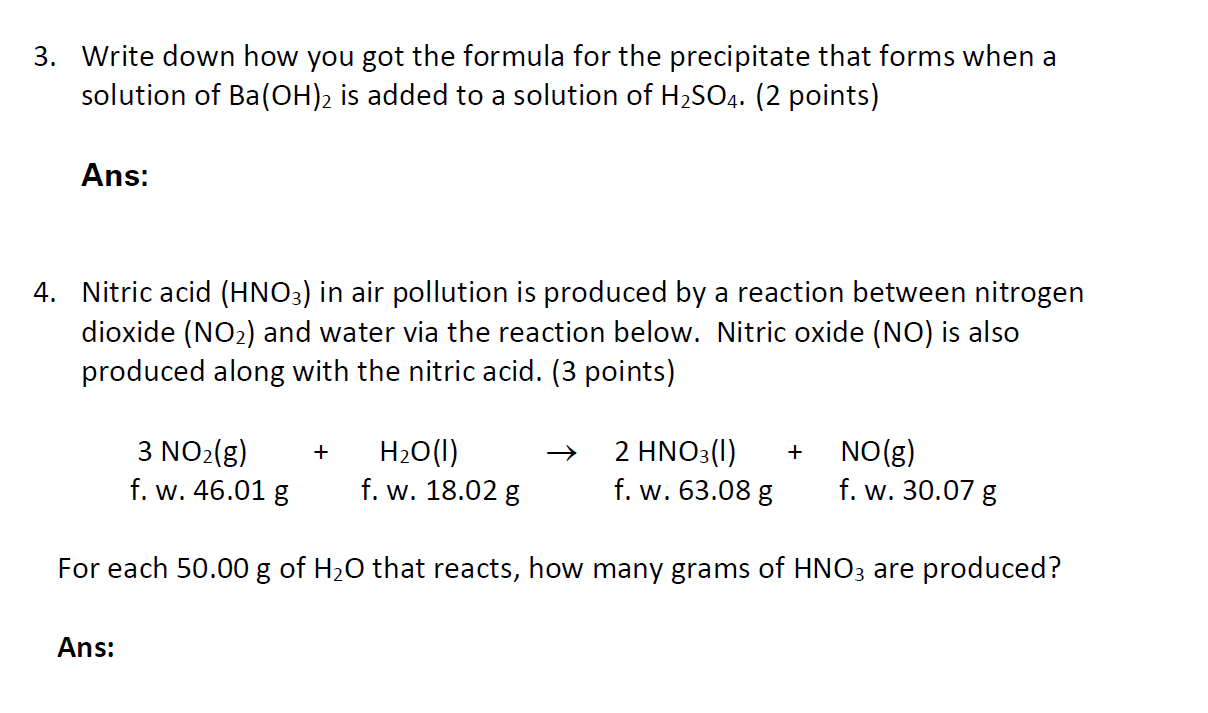
3. Write down how you got the formula for the precipitate that forms when a solution of Ba(OH)2 is added to a solution of H2SO4. (2 points) Ans: 4. Nitric acid (HNO3) in air pollution is produced by a reaction between nitrogen dioxide (NO2) and water via the reaction below. Nitric oxide (NO) is also produced along with the nitric acid. (3 points) + + 3 NO2(g) f. w. 46.01 g H2O(1) f. w. 18.02 g 2 HNO3(1) f. w. 63.08 g NO(g) f. w. 30.07 g For each 50.00 g of H20 that reacts, how many grams of HNO3 are produced? Ans
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


