Question: i need help with this please its an easy lab but im not sure about what im doing so if you guys can help me
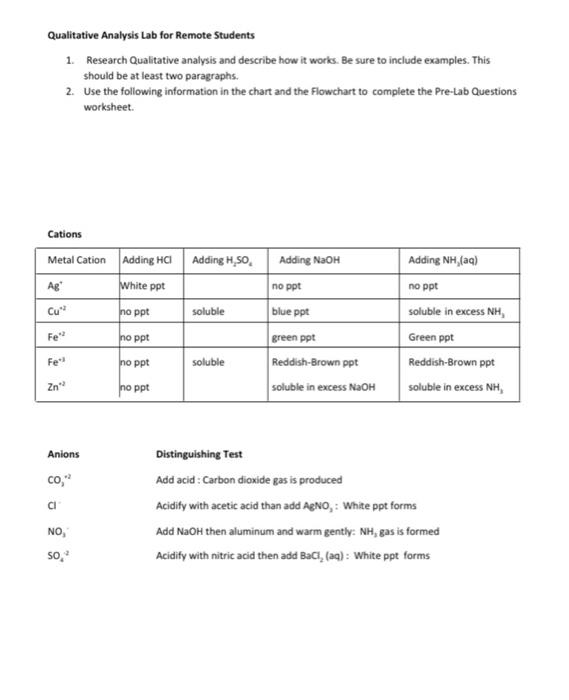
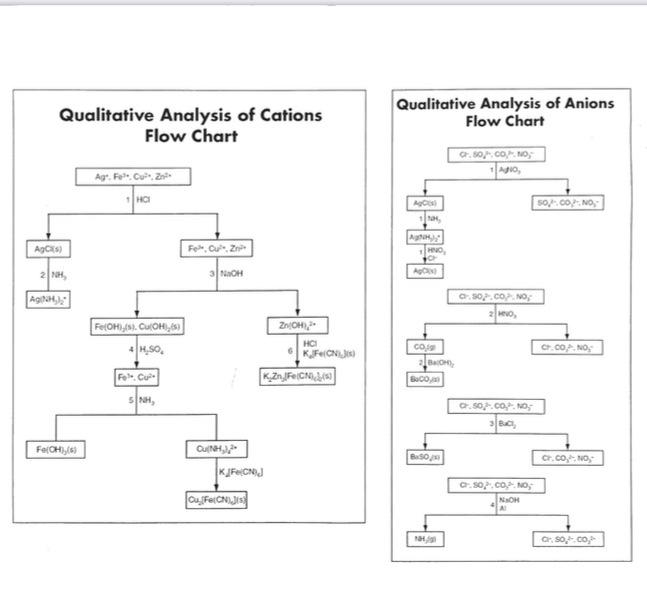

Qualitative Analysis Lab for Remote Students 1. Research Qualitative analysis and describe how it works. Be sure to include examples. This should be at least two paragraphs. 2. Use the following information in the chart and the Flowchart to complete the Pre-Lab Questions worksheet. Adding NaOH Cations Metal Cation Adding HCI Adding H,SO White ppt no ppt soluble no ppt blue ppt Adding NH(aq) no ppt soluble in excess NH Green ppt Reddish-Brown ppt soluble in excess NH Fe no ppt Fe no ppt soluble green ppt Reddish-Brown ppt soluble in excess NaOH Zn no ppt Anions CO," ci Distinguishing Test Add acid : Carbon dioxide gas is produced Acidity with acetic acid than add AgNO, : White ppt forms Add NaOH then aluminum and warm gently: NH, gas is formed Acidify with nitric acid then add BaC, (aa) : White ppt forms NO, 50, Qualitative Analysis of Cations Flow Chart Qualitative Analysis of Anions Flow Chart 0.50..CO.NO Agr. Focus HCI 507.00 NO AgC) Fleur TO 21 3 MOH AGON A p.) 0.507.CO.NO 20 FOR, CU OH,CO 4,50 Zn(OH)2 HCI KFC. KZn,FICNA360 C.CO.NO CO BOH SCO, Focus 5 0.50.CO.NO aa Fe[OH),(6) B.SO Cult, Kleiched C.CO.NO CufferCJI C-50..CO.NO NOH 10 C. SOCO Pre-Lab Questions Use the flow charts on the previous page to answer the following questions. In cach question, a test is carried out to determine the presence or absence of several ions. Only those listed may be present. State if the tests indicate if each ion is present, absent, or undetermined. 1. Test for Ag. Cu?". Fe Some 6 M HCl is added to a solution that may contain the three ions. A white precipitate forms. lons present Tons absent: Ions undetermined 2. Test for Cu?". Ag and Zn?" Some 6 M HCl is added to a solution that may contain the three ions. No precipitate forms. The addition of 6 M NaOH until the solution is basic results in no formation of precipitate. lons present fons absent Ions undetermined: 3. Test for Cu?". Fe" and Zn? 6 M NaOH is added to a clear solution that may contain the three ions until the solution is basic. A dark precipitate forms. The precipitate totally dissolves in 6 M H2SO4. The addition of 6 M NH, to this acidic solution until it is basic results in a clear solution containing a dark precipitate. The resulting dark precipitate completely dissolves in 6 M H2SO4 lons present lons absent: Tons undetermined: 4. Test for Co, and cr Some 6 M HCl is added to the solution that may contain the above ions. Formation of bubbles is noted as the solution is heated. Ions present lons absent: lons undetermined: 5. Test for Cr, so.co,?, and NO, Addition of AgNO, causes no precipitate to form. Addition of BaCl, to fresh solution also causes no precipitate to form. Tons present lons absent Ions undetermined: Only one of each of the following pairs of reactants undergoes a reaction. Complete and balance the equation for the reaction that occurs. 6. NaCl(a) + BaCl2(g) Na2SO4 (aq) + BaCl26) 7. NO (40) + OH + Alo + OH () + Alo) + CI) Ag (0) + C) cos? 8. K.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


