Question: 3.56. There are two ways to write the equation for the combustion of ethane: C2H6(g)+27O2(g)2C2H6(g)+7O2(g)3H2O(g)+2CO2(g)6H2O(g)+4CO2(g) Do these two different ways of writing the equation affect
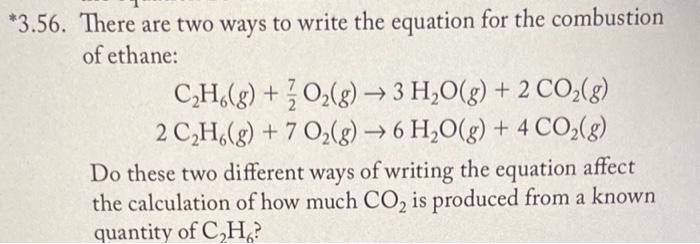
3.56. There are two ways to write the equation for the combustion of ethane: C2H6(g)+27O2(g)2C2H6(g)+7O2(g)3H2O(g)+2CO2(g)6H2O(g)+4CO2(g) Do these two different ways of writing the equation affect the calculation of how much CO2 is produced from a known quantity of C2H6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


