Question: 36 What is the order in each reactant and the overall order for a reaction that has the following rate law? (a) rate =k[O3][NO]2 (b)
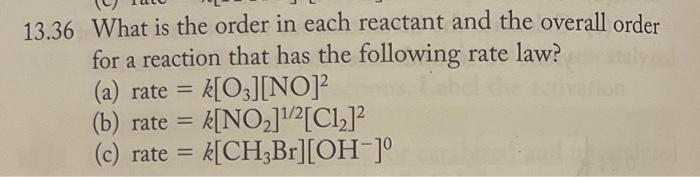
36 What is the order in each reactant and the overall order for a reaction that has the following rate law? (a) rate =k[O3][NO]2 (b) rate =k[NO2]1/2[Cl2]2 (c) rate =k[CH3Br][OH]0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


