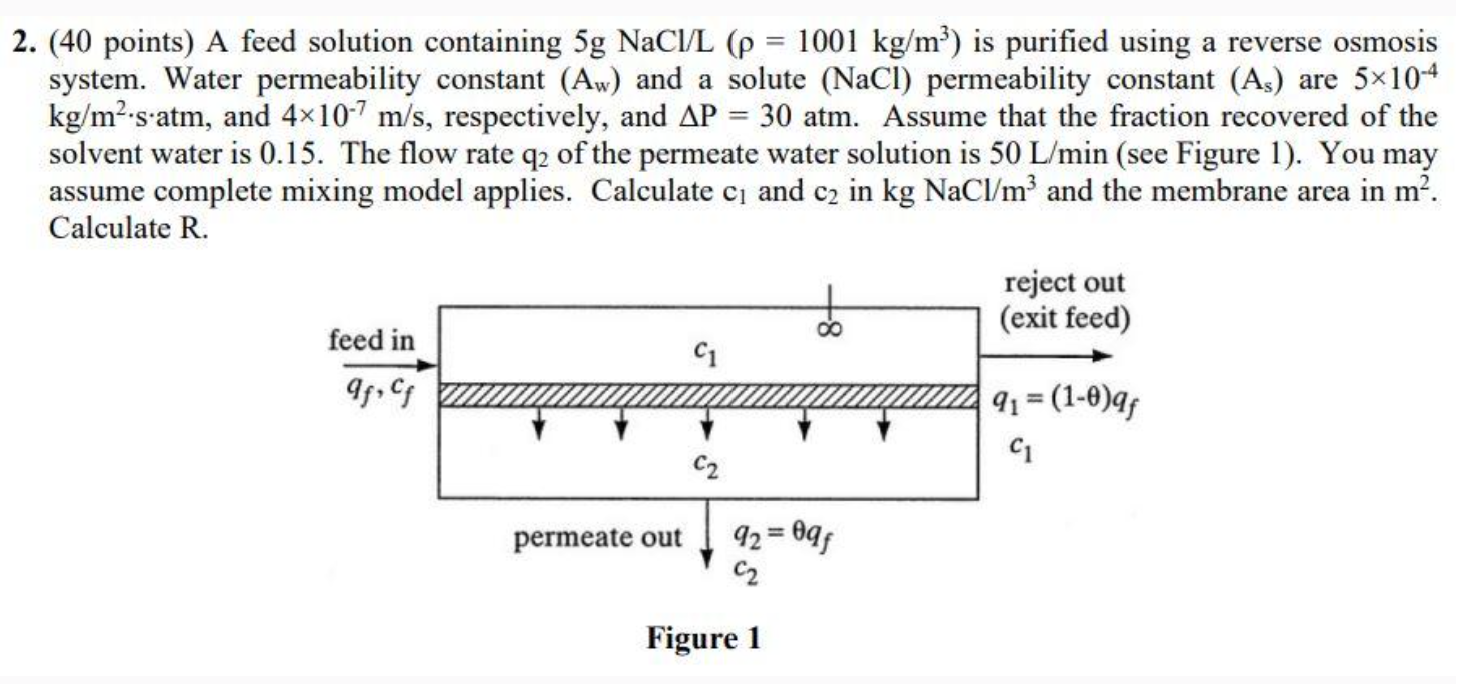
Question: ( 4 0 points ) A feed solution containing 5 gNaC l L ( = 1 0 0 1 k g m 3 ) is
points A feed solution containing gNaC is purified using a reverse osmosis system. Water permeability constant and a solute permeability constant are and respectively, and atm. Assume that the fraction recovered of the solvent water is The flow rate of the permeate water solution is see Figure You may assume complete mixing model applies. Calculate and in kgNaC and the membrane area in Calculate R
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


