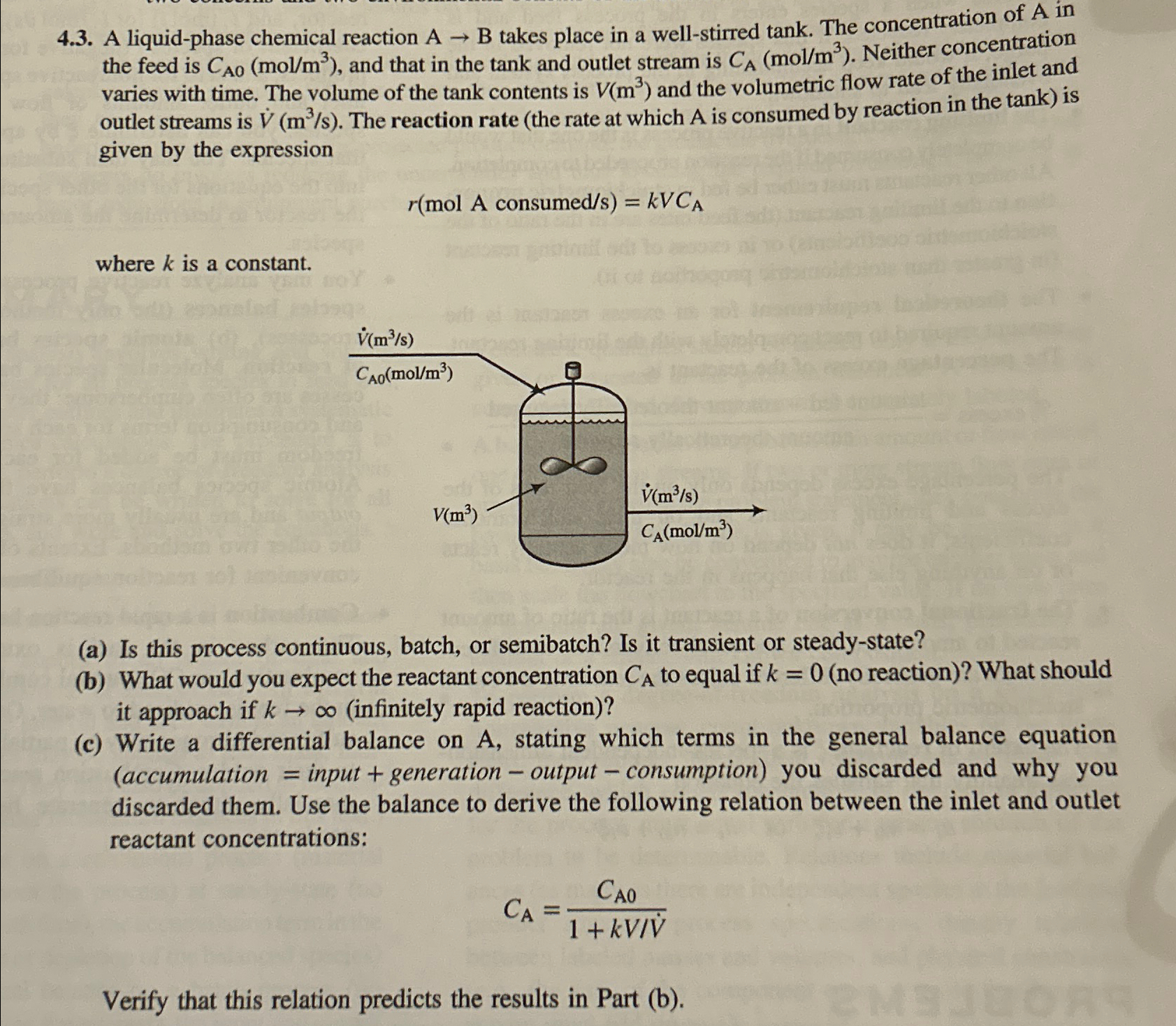
Question: 4 . 3 . A liquid - phase chemical reaction A B takes place in a well - stirred tank. The concentration of A in
A liquidphase chemical reaction takes place in a wellstirred tank. The concentration of in the feed is and that in the tank and outlet stream is Neither concentration varies with time. The volume of the tank contents is and the volumetric flow rate of the inlet and outlet streams is The reaction rate the rate at which is consumed by reaction in the tank is given by the expression
consume
where is a constant.
a Is this process continuous, batch, or semibatch? Is it transient or steadystate?
b What would you expect the reactant concentration to equal if no reaction What should it approach if infinitely rapid reaction
c Write a differential balance on A stating which terms in the general balance equation accumulation input generation output consumption
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


