Question: 4 4. At a given temperature, Kc = 6.0 for the reaction Cl:(8) + F2(g) 2 CIF(B) If the initial concentrations of CI (g) =
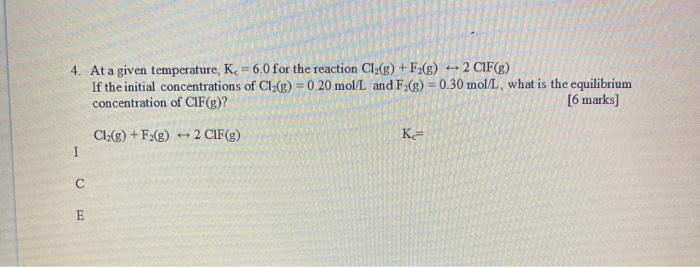
4 4. At a given temperature, Kc = 6.0 for the reaction Cl:(8) + F2(g) 2 CIF(B) If the initial concentrations of CI (g) = 0.20 mol/L and F.(g) =0.30 mol/l what is the equilibrium concentration of CIF(g)? [6 marks) Cl2(g) + F2(g) + 2 CIF(e) K= E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


