Question: 4. a. (8 points) Explain how you could separate an equimolar mixture of Compound X, Y and Z. (a flow chart). NH2 OH Compound X
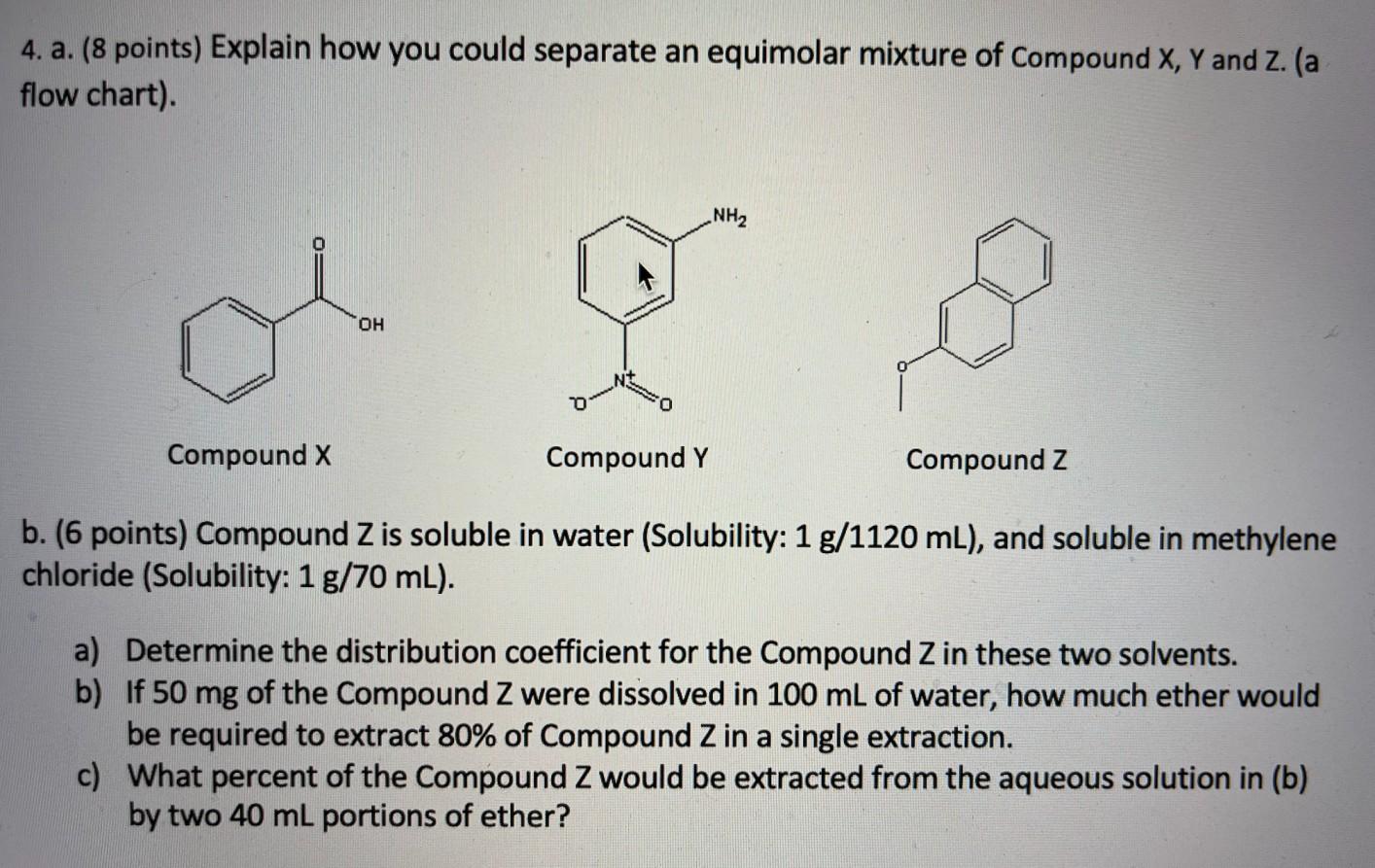
4. a. (8 points) Explain how you could separate an equimolar mixture of Compound X, Y and Z. (a flow chart). NH2 OH Compound X Compound Y Compound Z b. (6 points) Compound Z is soluble in water (Solubility: 1 g/1120 mL), and soluble in methylene chloride (Solubility: 1 g/70 mL). a) Determine the distribution coefficient for the Compound Z in these two solvents. b) If 50 mg of the Compound Z were dissolved in 100 mL of water, how much ether would be required to extract 80% of Compound Z in a single extraction. c) What percent of the Compound Z would be extracted from the aqueous solution in (b) by two 40 mL portions of ether
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


