Question: 4. A weak acid solution is made up at 0.001 M total concentration of the acid, HA, and it is found that the HA is
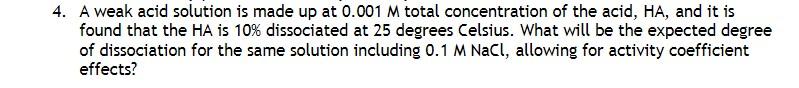
4. A weak acid solution is made up at 0.001 M total concentration of the acid, HA, and it is found that the HA is 10% dissociated at 25 degrees Celsius. What will be the expected degree of dissociation for the same solution including 0.1 M NaCl, allowing for activity coefficient effects
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


