Question: 4. Electroplating is a process used to put a metal coating onto an object, in order to protect and preserve it. In this case, the
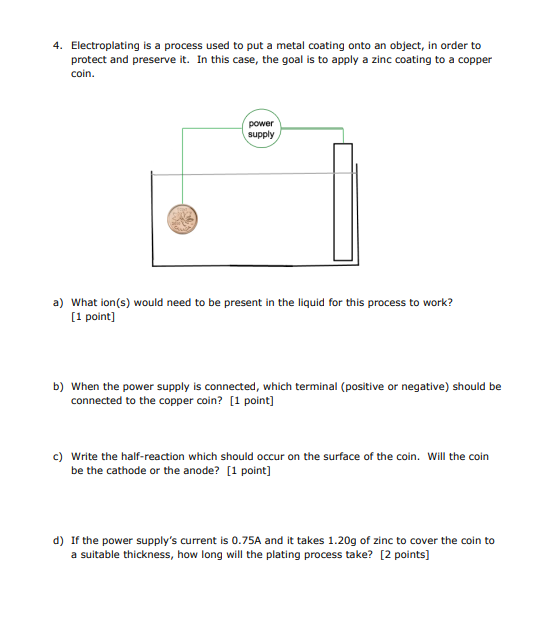
4. Electroplating is a process used to put a metal coating onto an object, in order to protect and preserve it. In this case, the goal is to apply a zinc coating to a copper coin. a) What ion(s) would need to be present in the liquid for this process to work? [1 point ] b) When the power supply is connected, which terminal (positive or negative) should be connected to the copper coin? [1 point] c) Write the half-reaction which should occur on the surface of the coin. Will the coin be the cathode or the anode? [1 point] d) If the power supply's current is 0.75A and it takes 1.20g of zinc to cover the coin to a suitable thickness, how long will the plating process take? [2 points]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


