Question: 4. In the process shown below, the container is insulated and the gas is ideal. a) Show that this process is isothermal (T2 = T)
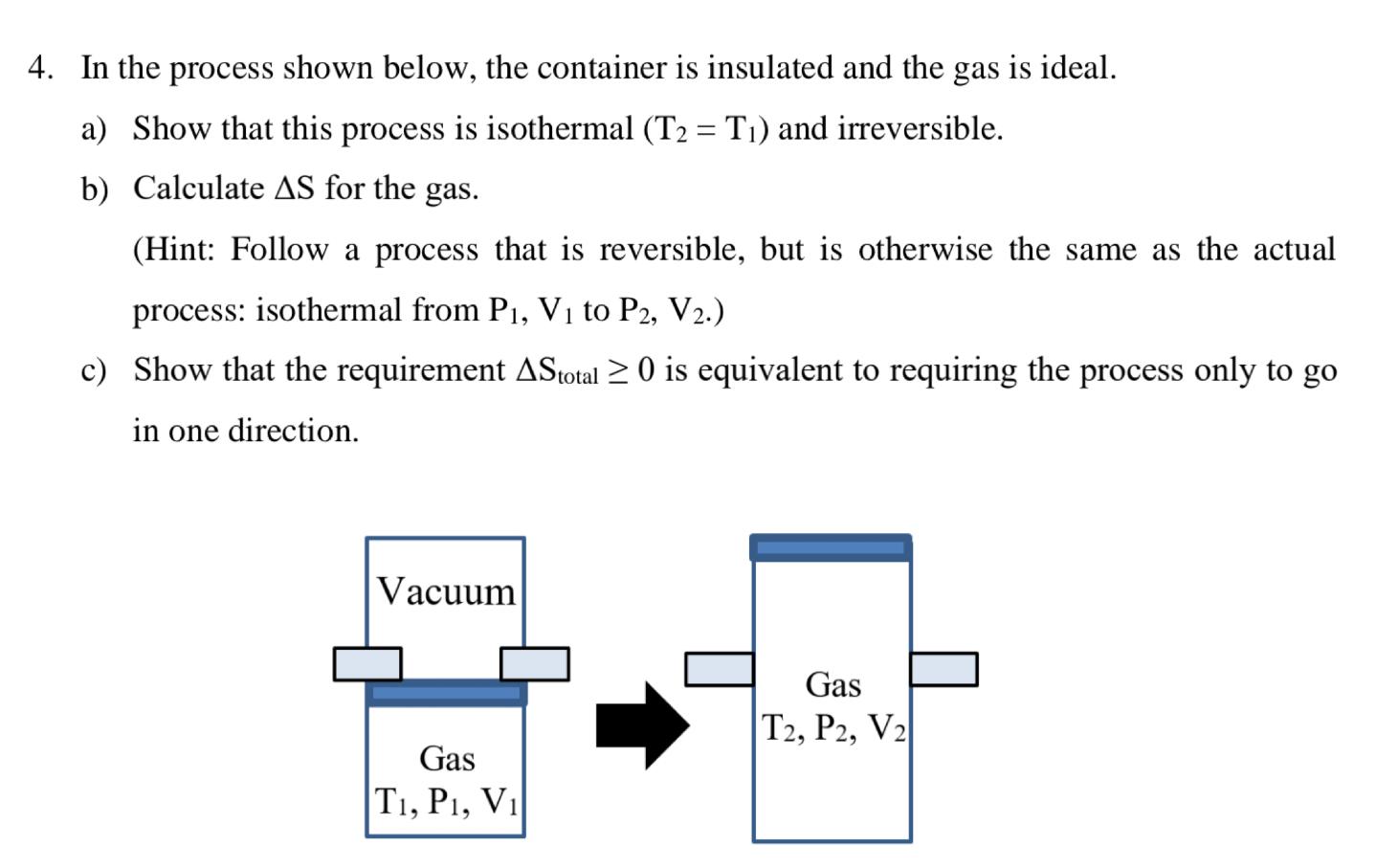
4. In the process shown below, the container is insulated and the gas is ideal. a) Show that this process is isothermal (T2 = T) and irreversible. b) Calculate AS for the gas. (Hint: Follow a process that is reversible, but is otherwise the same as the actual process: isothermal from P1, Vi to P2, V2.) c) Show that the requirement AStotal > 0 is equivalent to requiring the process only to go in one direction. , Vacuum Gas T2, P2, V2 Gas T, P1, Vi
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


