Question: Please Explain Istmes menure Tu The cyclic process shown at the right is a Carnot cycle, except that both isothermal steps are irreversible. So, the
Please Explain
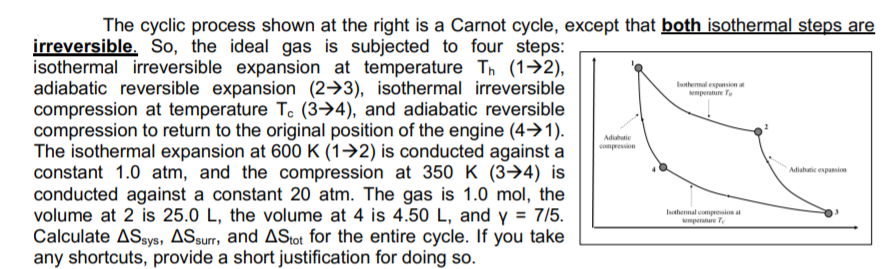
Istmes menure Tu The cyclic process shown at the right is a Carnot cycle, except that both isothermal steps are irreversible. So, the ideal gas is subjected to four steps: isothermal irreversible expansion at temperature Th (12), adiabatic reversible expansion (23), isothermal irreversible compression at temperature T. (34), and adiabatic reversible compression to return to the original position of the engine (4+1). The isothermal expansion at 600 K (1+2) is conducted against a constant 1.0 atm, and the compression at 350 K (3-4) is conducted against a constant 20 atm. The gas is 1.0 mol, the volume at 2 is 25.0 L, the volume at 4 is 4.50 L, and y = 7/5. Calculate ASsys, ASsurr, and AStot for the entire cycle. If you take any shortcuts, provide a short justification for doing so. Adidati Adiabatic espai Internal com rele
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


