Question: I'm only stuck on question 2. How do I know which way the signs for the work done on the gas? I often get confused
I'm only stuck on question 2. How do I know which way the signs for the work done on the gas? I often get confused by the convention of work done on the gas to work done to the gas. Also what do they mean when they say what does this cyclic process accomplish?
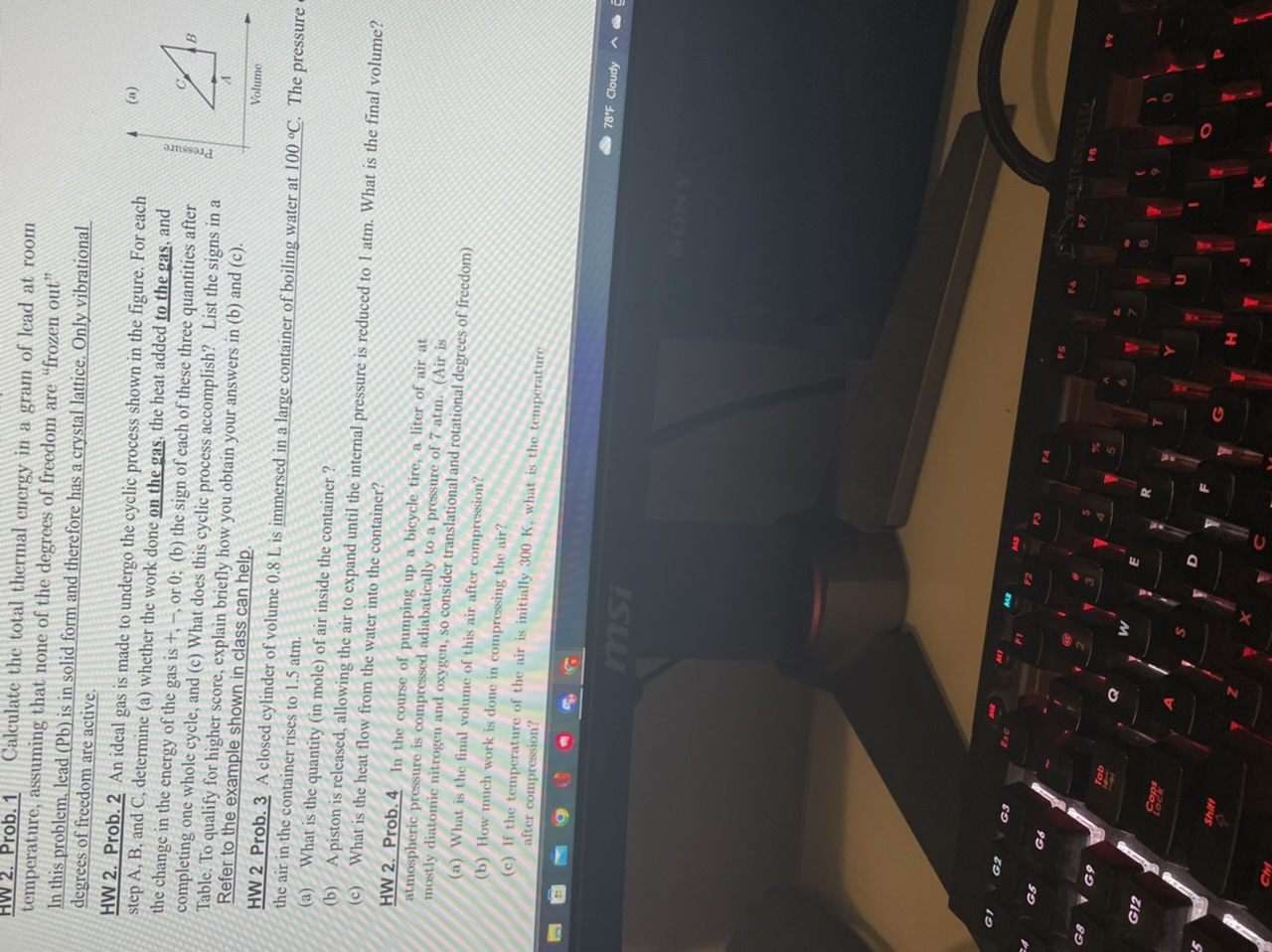
2. Prob. 1 Calculate the total thermal energy in a gram of lead at room temperature, assuming that none of the degrees of freedom are "frozen out" In this problem, lead (Pb) is in solid form and therefore has a crystal lattice. Only vibrational degrees of freedom are active. (a) HW 2. Prob. 2 An ideal gas is made to undergo the cyclic process shown in the figure. For each step A, B, and C, determine (a) whether the work done on the gas, the heat added to the gas, and Pressure the change in the energy of the gas is +, -, or 0; (b) the sign of each of these three quantities after completing one whole cycle, and (c) What does this cyclic process accomplish? List the signs in a Table. To qualify for higher score, explain briefly how you obtain your answers in (b) and (c). Volume Refer to the example shown in class can help. HW 2 Prob. 3 A closed cylinder of volume 0.8 L is immersed in a large container of boiling water at 100 .C. The pressure the air in the container rises to 1.5 atm. (a) What is the quantity (in mole) of air inside the container ? (b) A piston is released, allowing the air to expand until the internal pressure is reduced to 1 atm. What is the final volume? (c) What is the heat flow from the water into the container? HW 2. Prob. 4 In the course of pumping up a bicycle tire, a liter of air at atmospheric pressure is compressed adiabatically to a pressure of 7 atm. (Air is mostly diatomic nitrogen and oxygen, so consider translational and rotational degrees of freedom) (a) What is the final volume of this air after compression? (b) How much work is done in compressing the air? (c) If the temperature of the air is initially 300 K, what is the temperature after compression? 9 78'F Cloudy ~ msi G? GS GA F7 F9 G8 G9 G12 COR Shift
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


