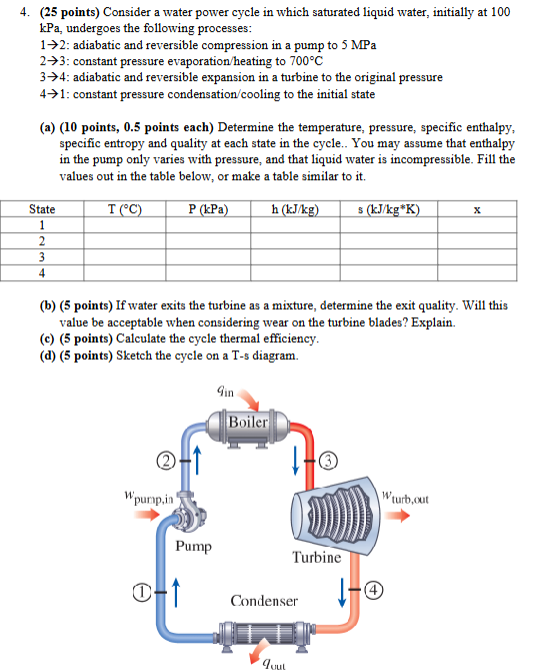
Question: 4 . ( ( mathbf { 2 5 } ) points ) Consider a water power cycle in which saturated liquid water,
mathbf points Consider a water power cycle in which saturated liquid water, initially at kPa undergoes the following processes:
rightarrow : adiabatic and reversible compression in a pump to MPa
rightarrow : constant pressure evaporationheating to circmathrmC
rightarrow : adiabatic and reversible expansion in a turbine to the original pressure
rightarrow : constant pressure condensationcooling to the initial state
a points, points each Determine the temperature, pressure, specific enthalpy, specific entropy and quality at each state in the cycle.. You may assume that enthalpy in the pump only varies with pressure, and that liquid water is incompressible. Fill the values out in the table below, or make a table similar to it
begintabularcccccc
hline State & mathrmTleftcircmathrmCright & mathrmPmathrmkPa & mathrmhmathrmkJmathrmkg & mathrmsmathrmkJmathrmkgmathrm~K & x
hline & & & & &
hline & & & & &
hline & & & & &
hline & & & & &
hline
endtabular
b points If water exits the turbine as a mixture, determine the exit quality. Will this value be acceptable when considering wear on the turbine blades? Explain.
c points Calculate the cycle thermal efficiency.
d points Sketch the cycle on a Ts diagram.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


