Question: (4 points) A liquid is under an extra external pressure P by some inert gas. Show that its vapor pressure p can be approximated by
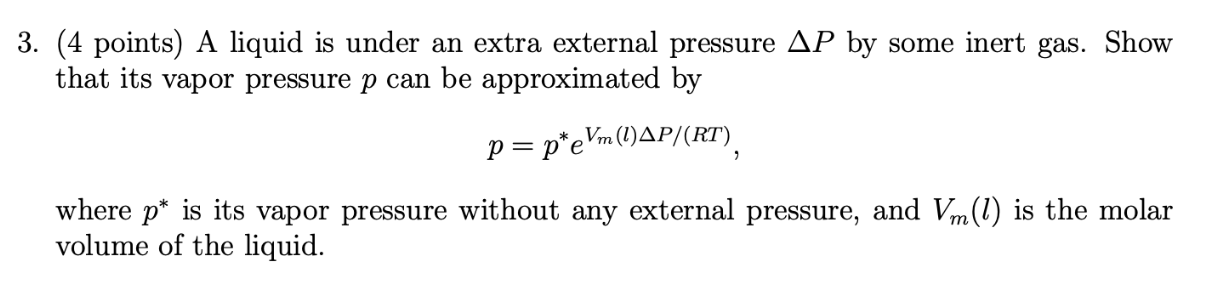
(4 points) A liquid is under an extra external pressure P by some inert gas. Show that its vapor pressure p can be approximated by p=peVm(l)P/(RT) where p is its vapor pressure without any external pressure, and Vm(l) is the molar volume of the liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


