Question: 4 points Savec A technologist collected the following calibration data for a Class A volumetric pipette: Table 1: Temperature (C) and mass (g) of water
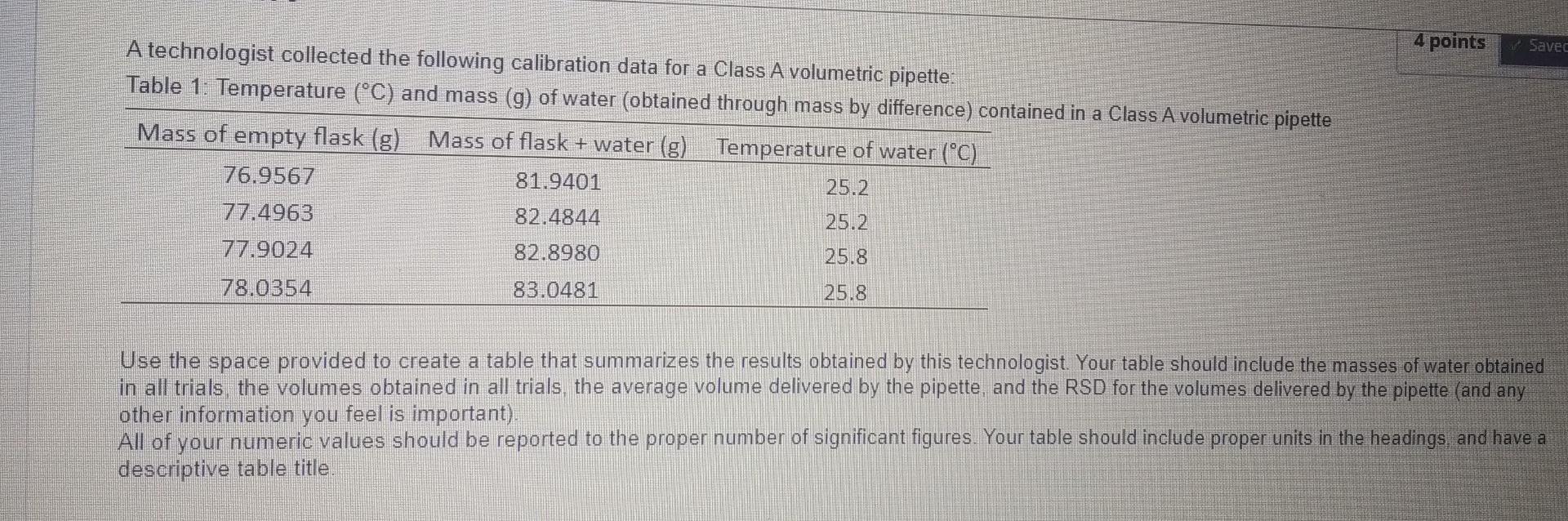

4 points Savec A technologist collected the following calibration data for a Class A volumetric pipette: Table 1: Temperature (C) and mass (g) of water (obtained through mass by difference) contained in a Class A volumetric pipette Mass of empty flask (g) Mass of flask + water (g) Temperature of water (C). 76.9567 81.9401 25.2. 77.4963 82.4844 25.2 77.9024 82.8980 25.8 78.0354 83.0481 25.8. Use the space provided to create a table that summarizes the results obtained by this technologist. Your table should include the masses of water obtained in all trials the volumes obtained in all trials, the average volume delivered by the pipette, and the RSD for the volumes delivered by the pipette (and any other information you feel is important). All of your numeric values should be reported to the proper number of significant figures. Your table should include proper units in the headings and have a descriptive table title. Based on the results given for the volumetric pipette above, write a discussion paragraph that describes what can be concluded about the calibration of this experiment (ie is this technologist using accurate and/or precise measuring technique?). Use mathematical evidence to justify your conclusion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


