Question: 4) Sodium Silver oxide decomposes based on given reaction below: Ag2O(s)Ag(s)+O2(g)(notbalanced) What is the total volume (as mL ) of produced oxygen by decomposition of
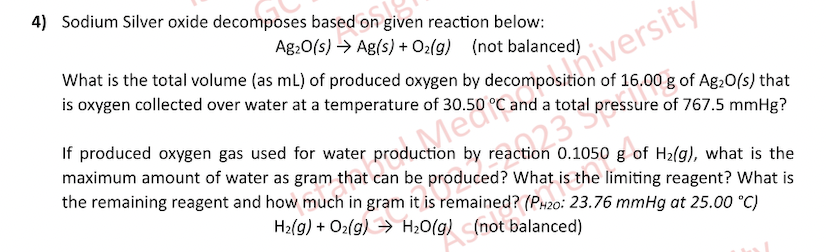
4) Sodium Silver oxide decomposes based on given reaction below: Ag2O(s)Ag(s)+O2(g)(notbalanced) What is the total volume (as mL ) of produced oxygen by decomposition of 16.00g of Ag2O(s ) that is oxygen collected over water at a temperature of 30.50C and a total pressure of 767.5mmHg ? If produced oxygen gas used for water production by reaction 0.1050g of H2(g), what is the maximum amount of water as gram that can be produced? What is the limiting reagent? What is the remaining reagent and how much in gram it is remained? (P P420:23.76mmHg at 25.00C ) H2(g)+O2(g)H2O(g)(notbalanced)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


