Question: When 8.750 g of butanol (C4H12O, MW = 76.14 g/mol) is combusted in the presence of excess oxygen at constant volume in a bomb
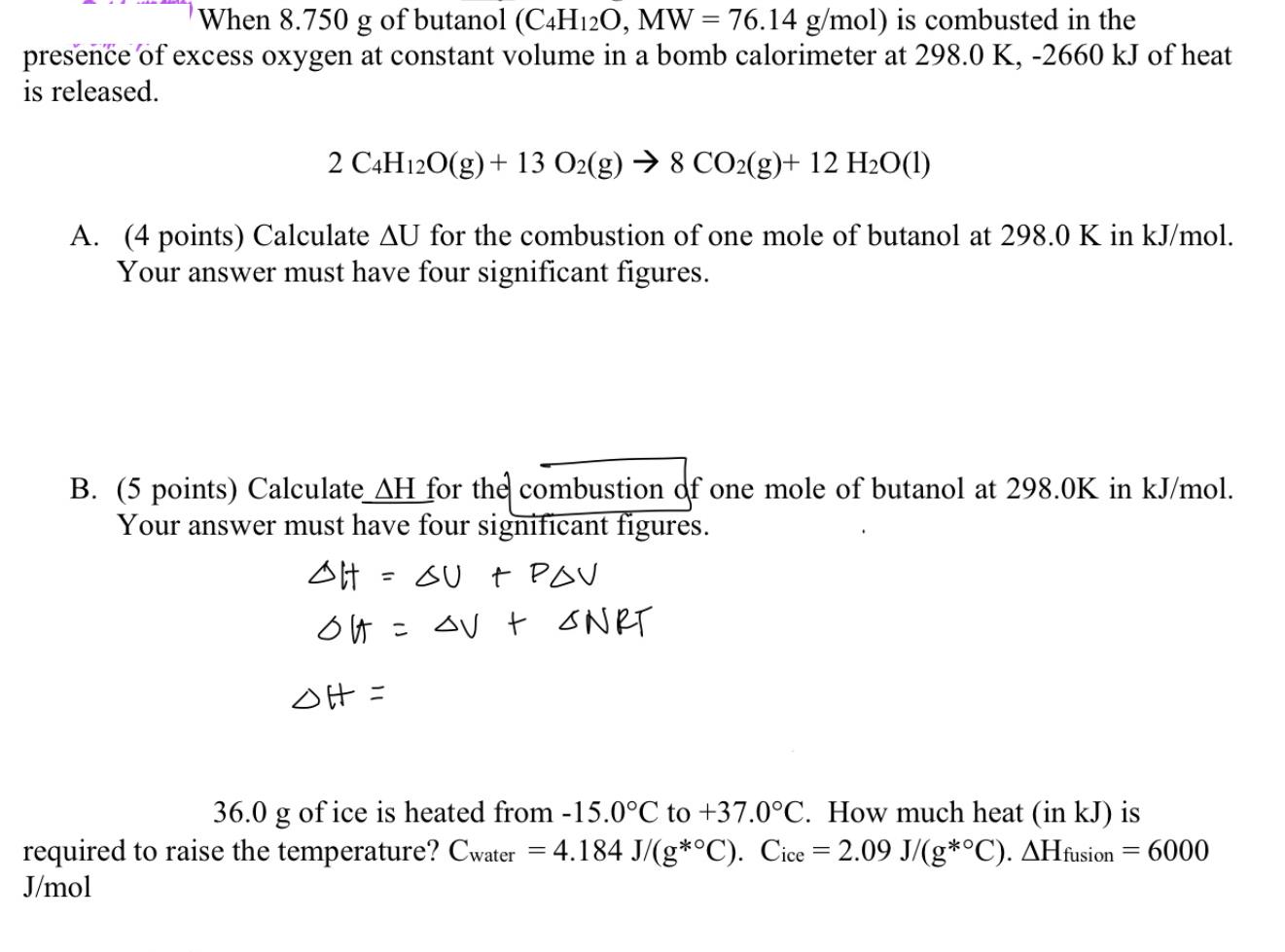
When 8.750 g of butanol (C4H12O, MW = 76.14 g/mol) is combusted in the presence of excess oxygen at constant volume in a bomb calorimeter at 298.0 K, -2660 kJ of heat is released. 2 C4H12O(g) + 13 O2(g) 8 CO2(g)+ 12 H2O(1) A. (4 points) Calculate AU for the combustion of one mole of butanol at 298.0 K in kJ/mol. Your answer must have four significant figures. B. (5 points) Calculate AH for the combustion of one mole of butanol at 298.0K in kJ/mol. Your answer must have four significant figures. OH = OU + PAV OH = V + ONRT OH = 36.0 g of ice is heated from -15.0C to +37.0C. How much heat (in kJ) is required to raise the temperature? Cwater = 4.184 J/(g*C). Cice = 2.09 J/(g*C). AHfusion = 6000 J/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


