Question: 4. Two polymers, each with NA = NB = N = 100 are blended at a temperature of T = 300 K. At this temperature,
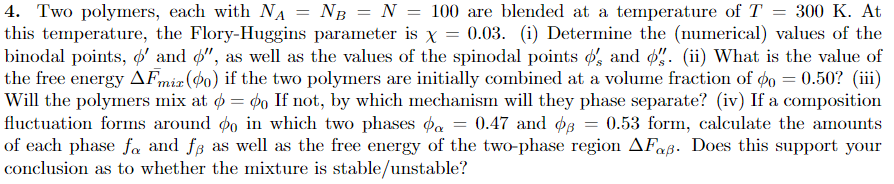
4. Two polymers, each with NA = NB = N = 100 are blended at a temperature of T = 300 K. At this temperature, the Flory-Huggins parameter is x = 0.03. (i) Determine the (numerical) values of the binodal points, ' and $", as well as the values of the spinodal points $'s and 6". (ii) What is the value of the free energy AFmiz (po) if the two polymers are initially combined at a volume fraction of po = 0.50? (iii) Will the polymers mix at $ = po If not, by which mechanism will they phase separate? (iv) If a composition fluctuation forms around po in which two phases oa = 0.47 and 08 = 0.53 form, calculate the amounts of each phase fa and fs as well as the free energy of the two-phase region AFa. Does this support your conclusion as to whether the mixture is stable/unstable? 4. Two polymers, each with NA = NB = N = 100 are blended at a temperature of T = 300 K. At this temperature, the Flory-Huggins parameter is x = 0.03. (i) Determine the (numerical) values of the binodal points, ' and $", as well as the values of the spinodal points $'s and 6". (ii) What is the value of the free energy AFmiz (po) if the two polymers are initially combined at a volume fraction of po = 0.50? (iii) Will the polymers mix at $ = po If not, by which mechanism will they phase separate? (iv) If a composition fluctuation forms around po in which two phases oa = 0.47 and 08 = 0.53 form, calculate the amounts of each phase fa and fs as well as the free energy of the two-phase region AFa. Does this support your conclusion as to whether the mixture is stable/unstable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


