Question: 4-17. Consecutive Reactions with Two Unknown Concentrations This is a variation on the steady reaction-diffusion system analyzed in Example 4.4-1 and Problem 4-16, in which
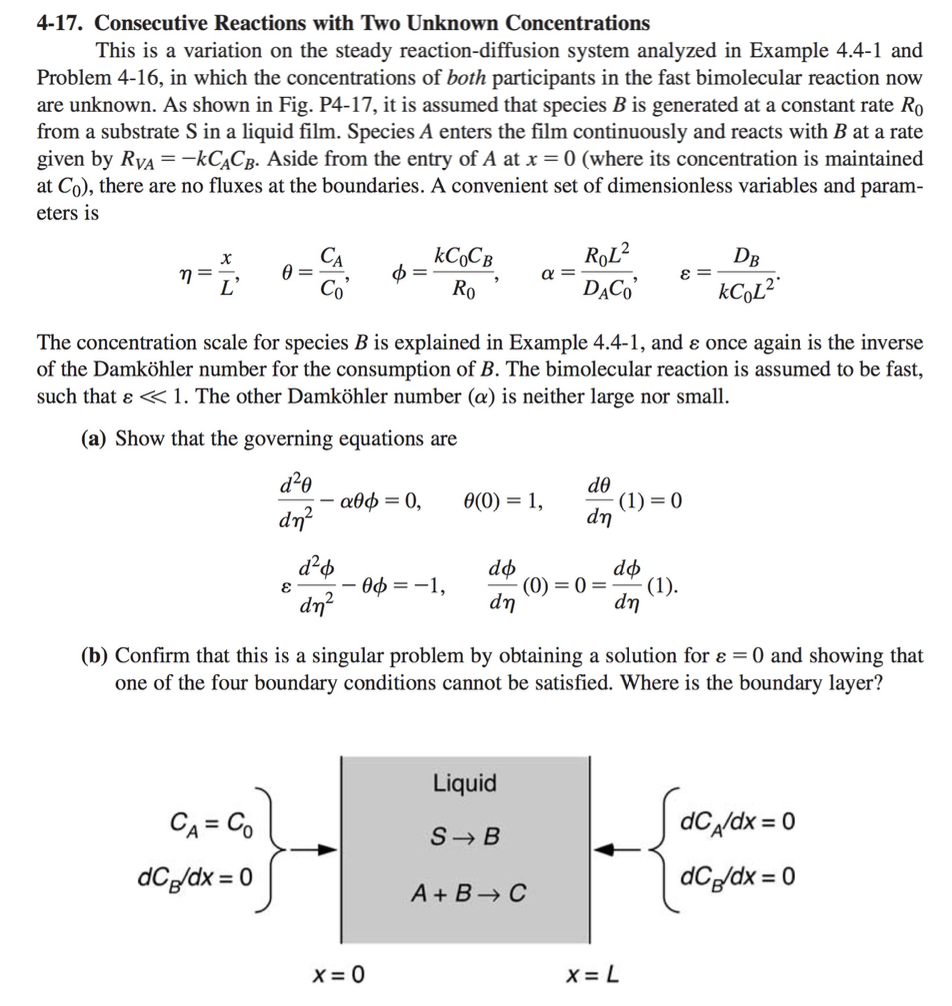
4-17. Consecutive Reactions with Two Unknown Concentrations This is a variation on the steady reaction-diffusion system analyzed in Example 4.4-1 and Problem 4-16, in which the concentrations of both participants in the fast bimolecular reaction now are unknown. As shown in Fig. P4-17, it is assumed that species B is generated at a constant rate Ro from a substrate S in a liquid film. Species A enters the film continuously and reacts with B at a rate given by Rva=-kCACB. Aside from the entry of A at x = 0 (where its concentration is maintained at Co), there are no fluxes at the boundaries. A convenient set of dimensionless variables and param- eters is X = L' = 0 kCCB Ro ROL? DACO E = DB kCol? The concentration scale for species B is explained in Example 4.4-1, and once again is the inverse of the Damkhler number for the consumption of B. The bimolecular reaction is assumed to be fast, such that
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


