Question: 4.9 Equation (4.38) indicates that an ideal PEM cell needs 30.35 g H, to pro- duce one kWh of electrical output. Suppose an electric car

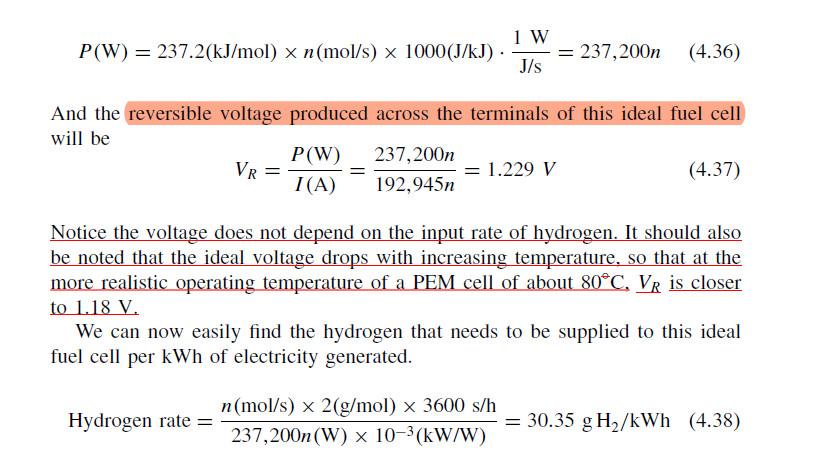
4.9 Equation (4.38) indicates that an ideal PEM cell needs 30.35 g H, to pro- duce one kWh of electrical output. Suppose an electric car can travel 10 miles per kWh. How much hydrogen would be needed to give the car a range of 300 miles if the source of electricity is an on-board fuel cell with an efficiency that is 50% of the ideal? P(W) = 237.2(kJ/mol) x n(mol/s) x 1000(J/kJ). 1 W J/s = 237,200n (4.36) And the reversible voltage produced across the terminals of this ideal fuel cell will be P(W) 237,200n VR = 1.229 V (4.37) I(A) 192,945n = Notice the voltage does not depend on the input rate of hydrogen. It should also be noted that the ideal voltage drops with increasing temperature, so that at the more realistic operating temperature of a PEM cell of about 80C. VR is closer to 1.18 V. We can now easily find the hydrogen that needs to be supplied to this ideal fuel cell per kWh of electricity generated. n(mol/s) x 2(g/mol) 3600 s/h Hydrogen rate = = 30.35 g H2/kWh (4.38) 237,200n(W) x 10-3(kW/W)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


