Question: Write a python code for this problem. write python code for this example. starting solution is given for pen paper mode, but I want a
Write a python code for this problem.
write python code for this example. starting solution is given for pen paper mode, but I want a python code for this example
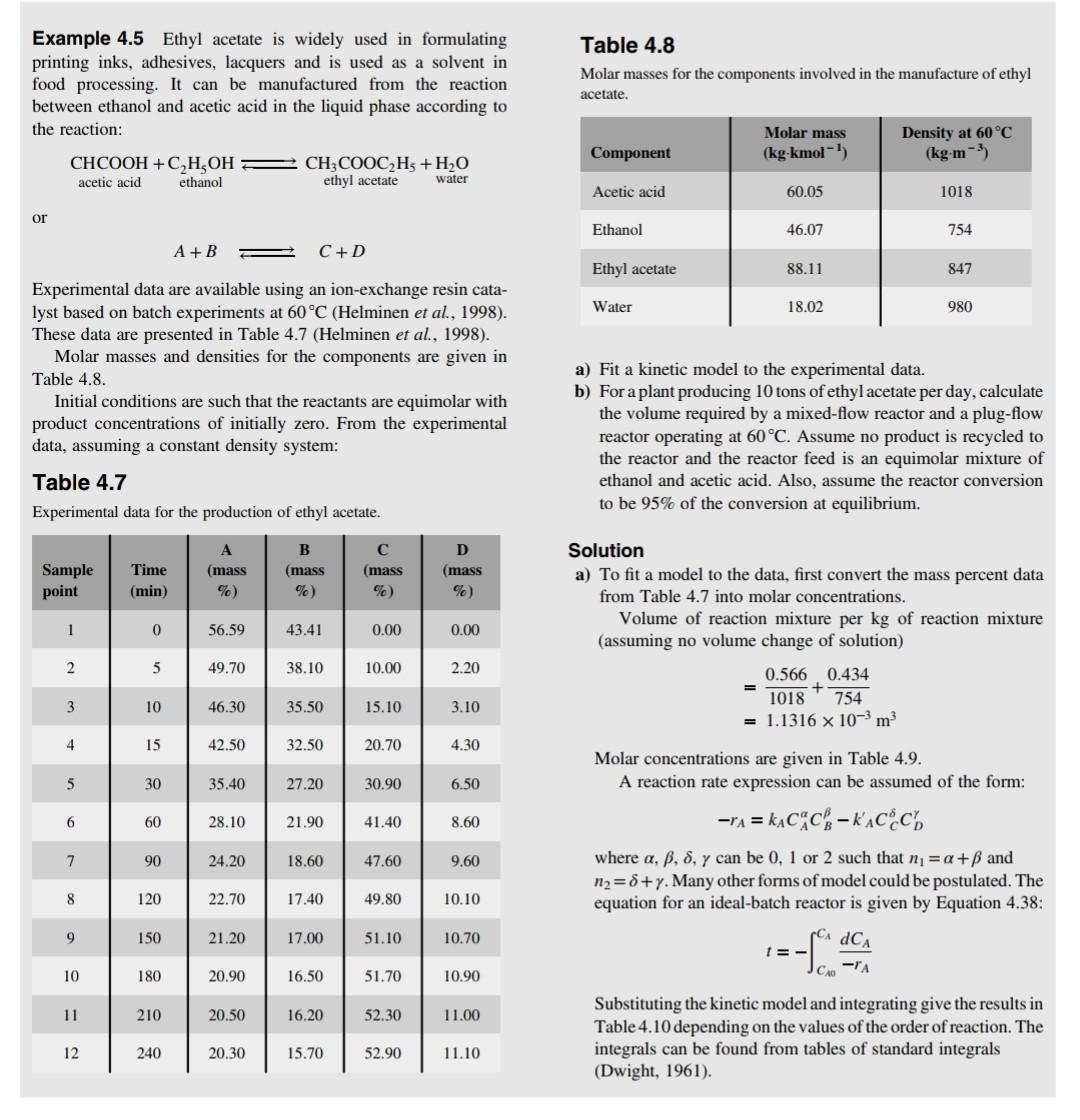
Example 4.5 Ethyl acetate is widely used in formulating Table 4.8 printing inks, adhesives, lacquers and is used as a solvent in food processing. It can be manufactured from the reaction Molar masses for the components involved in the manufacture of ethyl between ethanol and acetic acid in the liquid phase according to the reaction: aceticacidCHCOOH+ethanolC2H5OHethylacetateCH3COOC2H5+waterH2O or A+BC+D Experimental data are available using an ion-exchange resin catalyst based on batch experiments at 60C (Helminen et al., 1998). These data are presented in Table 4.7 (Helminen et al., 1998). Molar masses and densities for the components are given in Table 4.8. a) Fit a kinetic model to the experimental data. Initial conditions are such that the reactants are equimolar with b) For a plant producing 10 tons of ethyl acetate per day, calculate product concentrations of initially zero. From the experimental the volume required by a mixed-flow reactor and a plug-flow data, assuming a constant density system: reactor operating at 60C. Assume no product is recycled to the reactor and the reactor feed is an equimolar mixture of Table 4.7 ethanol and acetic acid. Also, assume the reactor conversion Experimental data for the production of ethyl acetate. to be 95% of the conversion at equilibrium. Solution a) To fit a model to the data, first convert the mass percent data from Table 4.7 into molar concentrations. Volume of reaction mixture per kg of reaction mixture (assuming no volume change of solution) =10180.566+7540.434=1.1316103m3 Molar concentrations are given in Table 4.9. A reaction rate expression can be assumed of the form: rA=kACACBkACCCD where ,,, can be 0,1 or 2 such that n1=+ and n2=+. Many other forms of model could be postulated. The equation for an ideal-batch reactor is given by Equation 4.38: t=CA0CArAdCA Substituting the kinetic model and integrating give the results in Table 4.10 depending on the values of the order of reaction. The integrals can be found from tables of standard integrals (Dwight, 1961). Example 4.5 Ethyl acetate is widely used in formulating Table 4.8 printing inks, adhesives, lacquers and is used as a solvent in food processing. It can be manufactured from the reaction Molar masses for the components involved in the manufacture of ethyl between ethanol and acetic acid in the liquid phase according to the reaction: aceticacidCHCOOH+ethanolC2H5OHethylacetateCH3COOC2H5+waterH2O or A+BC+D Experimental data are available using an ion-exchange resin catalyst based on batch experiments at 60C (Helminen et al., 1998). These data are presented in Table 4.7 (Helminen et al., 1998). Molar masses and densities for the components are given in Table 4.8. a) Fit a kinetic model to the experimental data. Initial conditions are such that the reactants are equimolar with b) For a plant producing 10 tons of ethyl acetate per day, calculate product concentrations of initially zero. From the experimental the volume required by a mixed-flow reactor and a plug-flow data, assuming a constant density system: reactor operating at 60C. Assume no product is recycled to the reactor and the reactor feed is an equimolar mixture of Table 4.7 ethanol and acetic acid. Also, assume the reactor conversion Experimental data for the production of ethyl acetate. to be 95% of the conversion at equilibrium. Solution a) To fit a model to the data, first convert the mass percent data from Table 4.7 into molar concentrations. Volume of reaction mixture per kg of reaction mixture (assuming no volume change of solution) =10180.566+7540.434=1.1316103m3 Molar concentrations are given in Table 4.9. A reaction rate expression can be assumed of the form: rA=kACACBkACCCD where ,,, can be 0,1 or 2 such that n1=+ and n2=+. Many other forms of model could be postulated. The equation for an ideal-batch reactor is given by Equation 4.38: t=CA0CArAdCA Substituting the kinetic model and integrating give the results in Table 4.10 depending on the values of the order of reaction. The integrals can be found from tables of standard integrals (Dwight, 1961)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


