Question: 4(a) Iodide ion is oxidized by hypochlorite ion in basic solution: I(aq)+ClO(aq)Cl(aq)+10(aq) In 1.00MNaOH at 25C, the iodide-ion concentration (equal to the ClOconcentration) at different
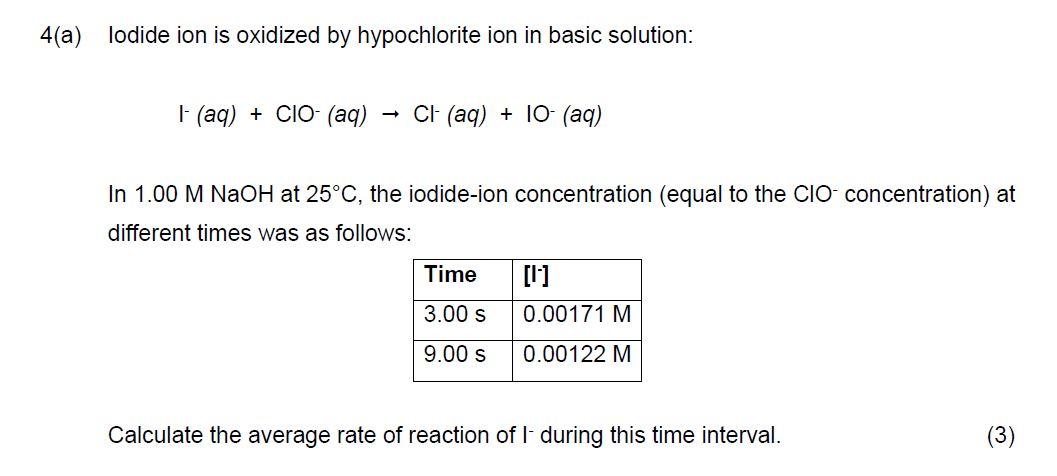
4(a) Iodide ion is oxidized by hypochlorite ion in basic solution: I(aq)+ClO(aq)Cl(aq)+10(aq) In 1.00MNaOH at 25C, the iodide-ion concentration (equal to the ClOconcentration) at different times was as follows: Calculate the average rate of reaction of I - during this time interval. (3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


