Question: 5. (15 points) A heat transfer unit is designed to use air at 150 C to activate an endothermic reaction. For this purpose, a pipe
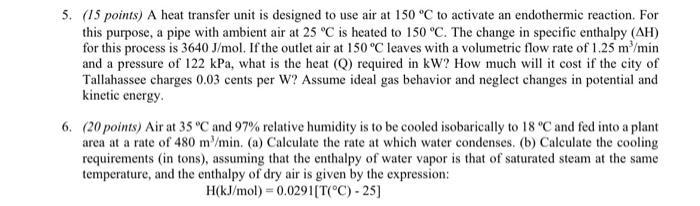
5. (15 points) A heat transfer unit is designed to use air at 150 C to activate an endothermic reaction. For this purpose, a pipe with ambient air at 25C is heated to 150 C. The change in specific enthalpy (AH) for this process is 3640 J/mol. If the outlet air at 150C leaves with a volumetric flow rate of 1.25 m/min and a pressure of 122 kPa, what is the heat (Q) required in kW? How much will it cost if the city of Tallahassee charges 0.03 cents per W? Assume ideal gas behavior and neglect changes in potential and kinetic energy 6. (20 points) Air at 35 C and 97% relative humidity is to be cooled isobarically to 18 C and fed into a plant area at a rate of 480 m/min. (a) Calculate the rate at which water condenses. (b) Calculate the cooling requirements (in tons), assuming that the enthalpy of water vapor is that of saturated steam at the same temperature, and the enthalpy of dry air is given by the expression: H(kJ/mol = 0.0291[T(C) -25)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


