Question: * 5 . 2 . 3 Phosgene gas is probably most famous for being the first toxic gas used offensively in World War I, but
Phosgene gas is probably most famous for being the first toxic gas used offensively in World War I, but it is also used extensively in the chemical
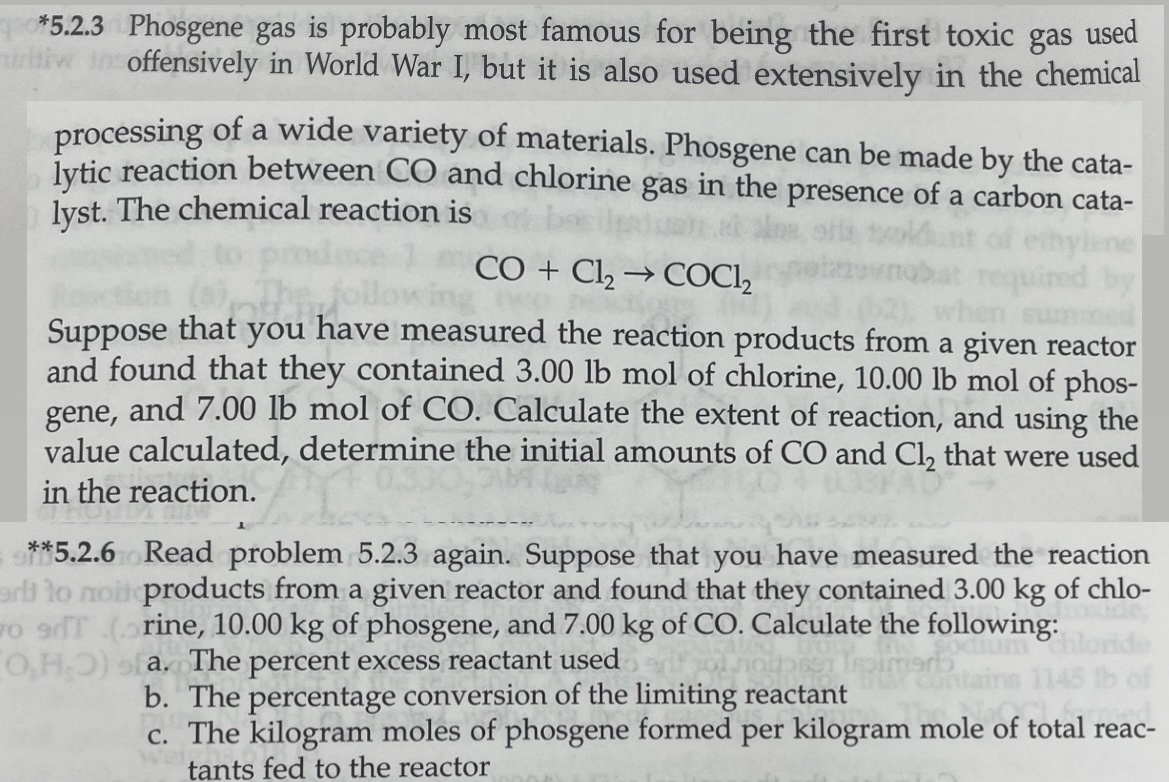
processing of a wide variety of materials. Phosgene can be made by the catalytic reaction between and chlorine gas in the presence of a carbon catalyst. The chemical reaction is
Suppose that you have measured the reaction products from a given reactor and found that they contained lbmol of chlorine, lbmol of phosgene, and lbmol of Calculate the extent of reaction, and using the value calculated, determine the initial amounts of and that were used in the reaction.
Read problem again. Suppose that you have measured the reaction products from a given reactor and found that they contained of chlorine, of phosgene, and of CO Calculate the following:
a The percent excess reactant used
b The percentage conversion of the limiting reactant
c The kilogram moles of phosgene formed per kilogram mole of total reactants fed to the reactor
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


