Question: 5 . 8 . A mixed flow reactor ( 2 m 2 ) processes an aqueous feed ( 1 0 0 liter / min )
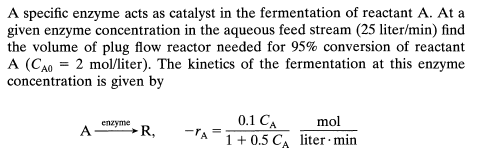
A mixed flow reactor m processes an aqueous feed litermin containing reactant A CAO mmolliter The reaction is reversible and represented by AFR PA CA CR mol liter min What is the equilibrium conversion and the actual conversion in the reactor?A specific enzyme acts as catalyst in the fermentation of reactant A At a
given enzyme concentration in the aqueous feed stream liter find
the volume of plug flow reactor needed for conversion of reactant
liter
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


