Question: A mixed flow reactor (2 m 3 ) processes an aqueous feed (100 liter/min) containing reactant A (C A0 = 100 mmol/liter). The reaction is
A mixed flow reactor (2 m3) processes an aqueous feed (100 liter/min) containing reactant A (CA0 = 100 mmol/liter). The reaction is reversible and represented by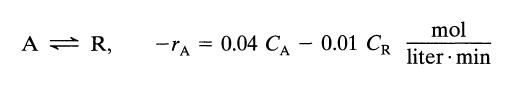
What is the equilibrium conversion and the actual conversion in the reactor?
A = R, -TA 0.04 CA - 0.01 CR mol liter min
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
To determine the equilibrium conversion and actual conversion in the mixed flow reactor we need to consider the reversible reaction and use the given ... View full answer

Get step-by-step solutions from verified subject matter experts


