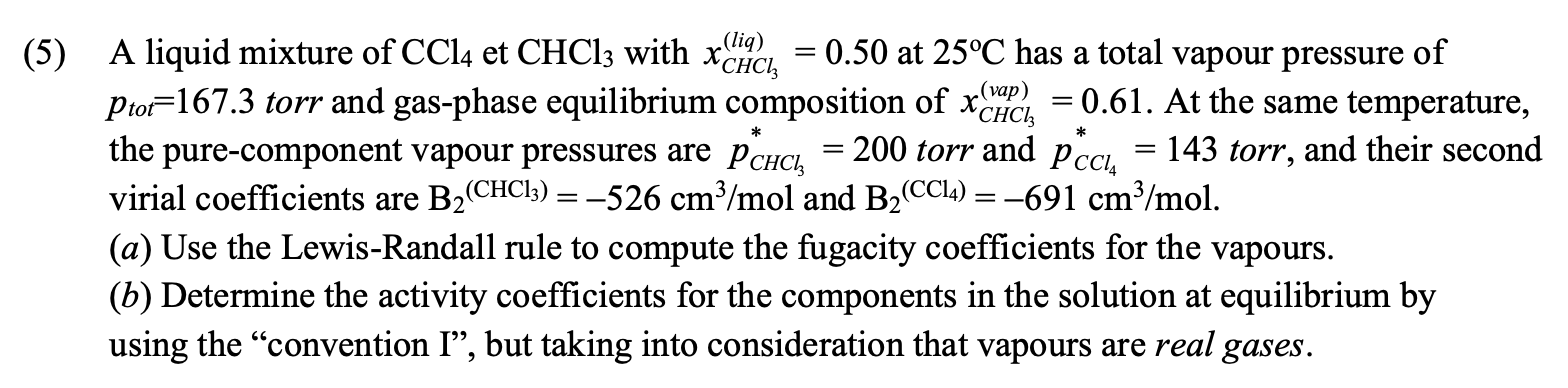
Question: ( 5 ) A liquid mixture of C C l 4 et C H C l 3 with x C H C l 3 (
A liquid mixture of et with at has a total vapour pressure of
torr and gasphase equilibrium composition of At the same temperature,
the purecomponent vapour pressures are torr and torr, and their second
virial coefficients are and
a Use the LewisRandall rule to compute the fugacity coefficients for the vapours.
b Determine the activity coefficients for the components in the solution at equilibrium by
using the "convention I", but taking into consideration that vapours are real gases.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


