Question: 5. (a) Why does carbon burning require higher temperatures than the triple-alpha process? [10] 10- (b) Two possible carbon-burning fusion reactions are as follows: 12C
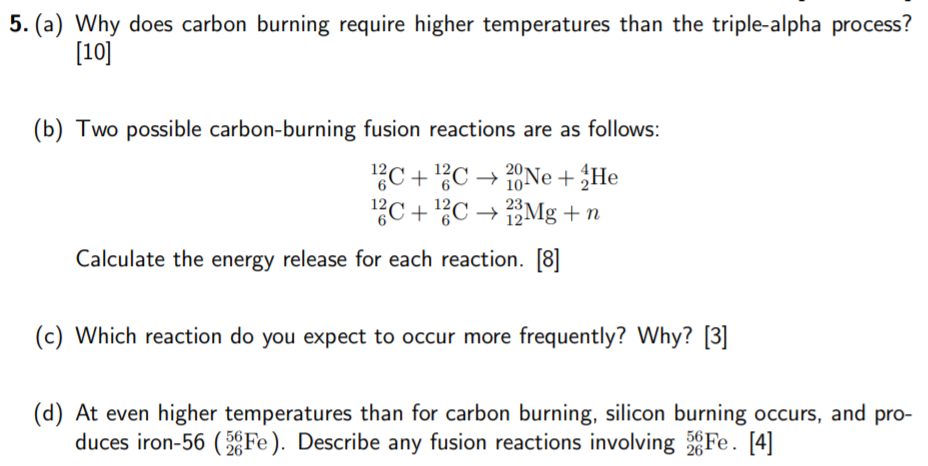
5. (a) Why does carbon burning require higher temperatures than the triple-alpha process? [10] 10- (b) Two possible carbon-burning fusion reactions are as follows: 12C + 12C 20Ne + He 13C + 12C 22 Mg +n Calculate the energy release for each reaction. [8] (c) Which reaction do you expect to occur more frequently? Why? [3] (d) At even higher temperatures than for carbon burning, silicon burning occurs, and pro- duces iron-56 (36Fe). Describe any fusion reactions involving 28 Fe. [4] 561 26
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


