Question: 5. An Arrhenius plot is given below for the gas phase reaction 2H1 + 02 + H202 + I2. -20 -21 -22 -23 y =
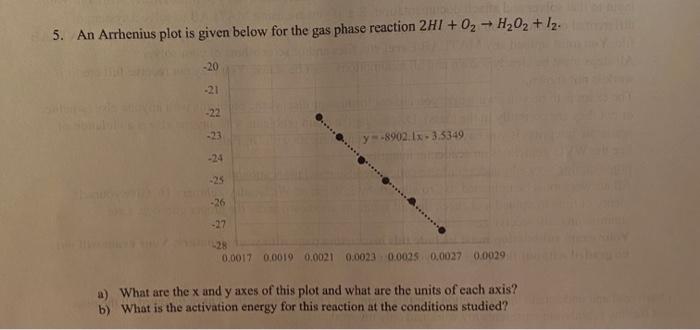
5. An Arrhenius plot is given below for the gas phase reaction 2H1 + 02 + H202 + I2. -20 -21 -22 -23 y = -8902:12 - 3.5349 -23 -26 -27 128 0.0017 0.0019 0,0021 0.0023 0.0025 0,0027 0.0029 a) What are the x and y axes of this plot and what are the units of each axis? b) What is the activation energy for this reaction at the conditions studied
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


