Question: a 1. A Levenspiel plot for a given reaction has a positive slope for all X. Would it be better to use a CSTR or
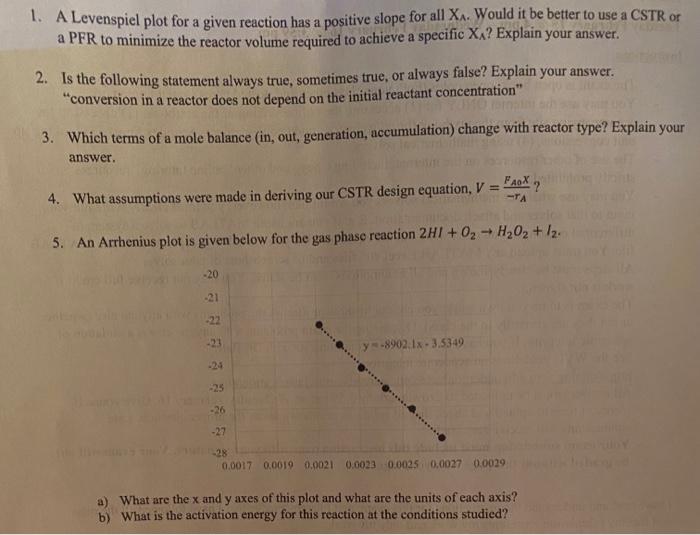
a 1. A Levenspiel plot for a given reaction has a positive slope for all X. Would it be better to use a CSTR or a PFR to minimize the reactor volume required to achieve a specific Xx? Explain your answer. 2. Is the following statement always true, sometimes true, or always false? Explain your answer. conversion in a reactor does not depend on the initial reactant concentration" 3. Which terms of a mole balance (in, out, generation, accumulation) change with reactor type? Explain your answer. ', 4. What assumptions were made in deriving our CSTR design equation, V = -TA 5. An Arrhenius plot is given below for the gas phase reaction 2H1 + 02 - H202 + 12. -20 21 y-8902.1x. 3.5349 -26 -27 28 0.0017 0.0019 0,0021 0.0023 0.0025 0,0027 0.0029 a) What are the x and y axes of this plot and what are the units of each axis? b) What is the activation energy for this reaction at the conditions studied
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


