Question: 5. An unknown compound has a formula of AX3. Using the Lewis structures draw below, draw the overall polarity of the molecule for each situation.
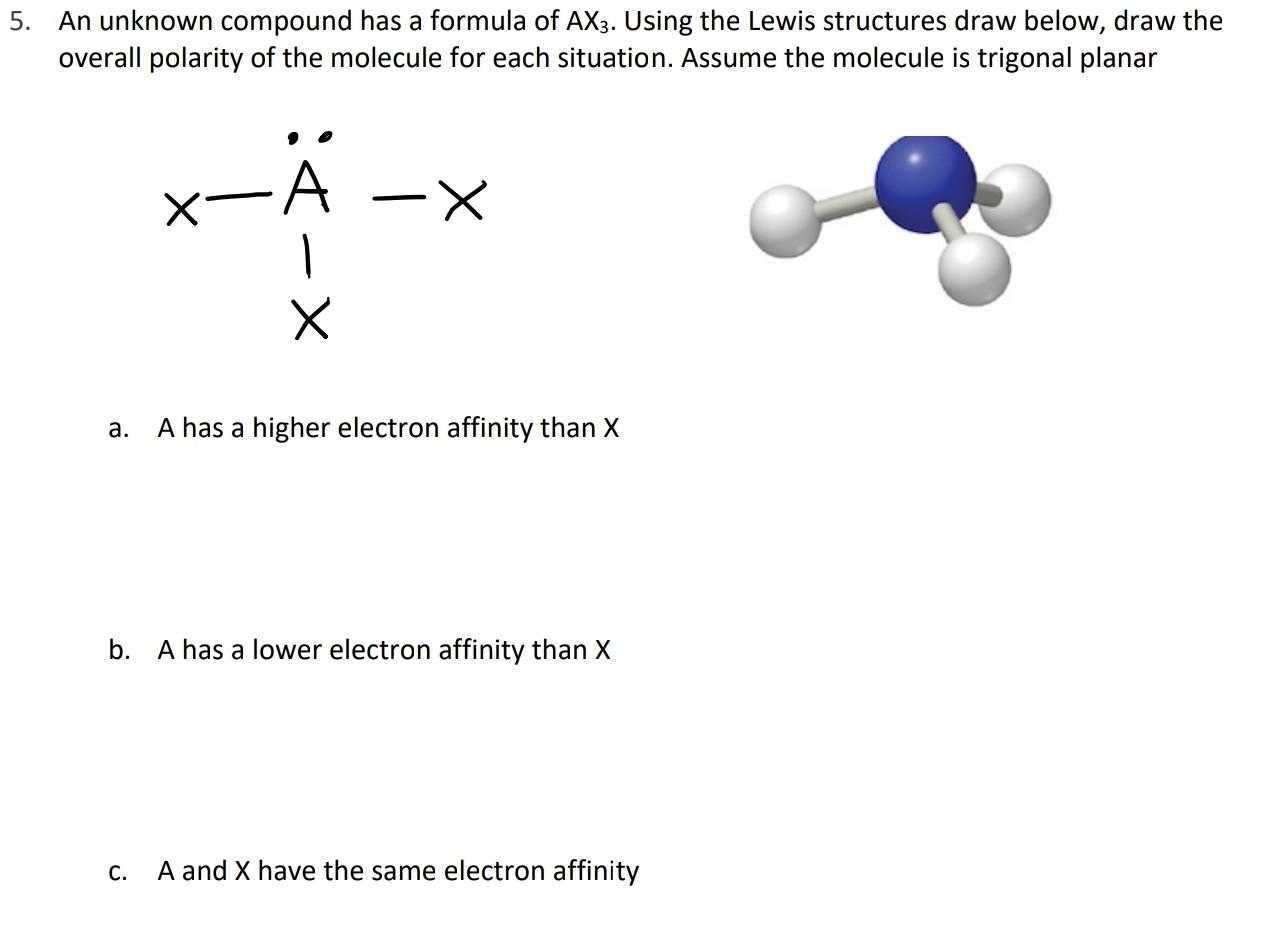
5. An unknown compound has a formula of AX3. Using the Lewis structures draw below, draw the overall polarity of the molecule for each situation. Assume the molecule is trigonal planar -A X a. A has a higher electron affinity than X b. A has a lower electron affinity than X C. A and X have the same electron affinity
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


