Question: 5. Compounds A and B have a 17.2kJ/mol difference in their heats of combustion. Redraw these compounds showing chair or boat conformations for all six-membered
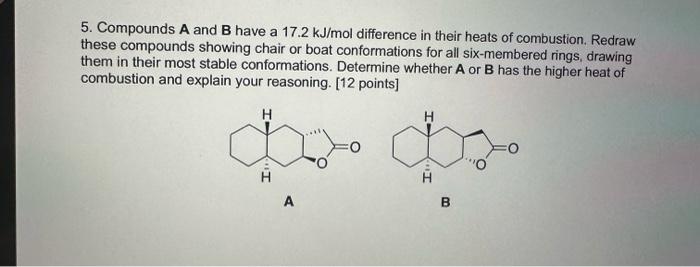
5. Compounds A and B have a 17.2kJ/mol difference in their heats of combustion. Redraw these compounds showing chair or boat conformations for all six-membered rings, drawing them in their most stable conformations. Determine whether A or B has the higher heat of combustion and explain your reasoning. [12 points] A B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


