Question: ( 5 pt ) Solid aluminum phosphate ( A l P O 4 ) is in equilibrium with its ions in solution: A l P
pt Solid aluminum phosphate is in equilibrium with its ions in solution:
with
Find the equilibrium concentration of phosphate ions in
pt Calculate the equilibrium concentration of dissolved oxygen in water in :
a pt at and atm ie sea level
b pt at and elevation
pt Suppose the gas above the soda in a bottle of soft drink is pure at a pressure of atm. Calculate concentration in in the aqueous phase ie the soda at
pt A sample of groundwater has of and Find the total hardness expressed in milliequivalents per liter meq and as How would this water be classified eg soft, hard, etc.
a pt Ignoring the contribution of and to alkalinity, what is the alkalinity as of
b pt Including the contribution of and find the alkalinity as of
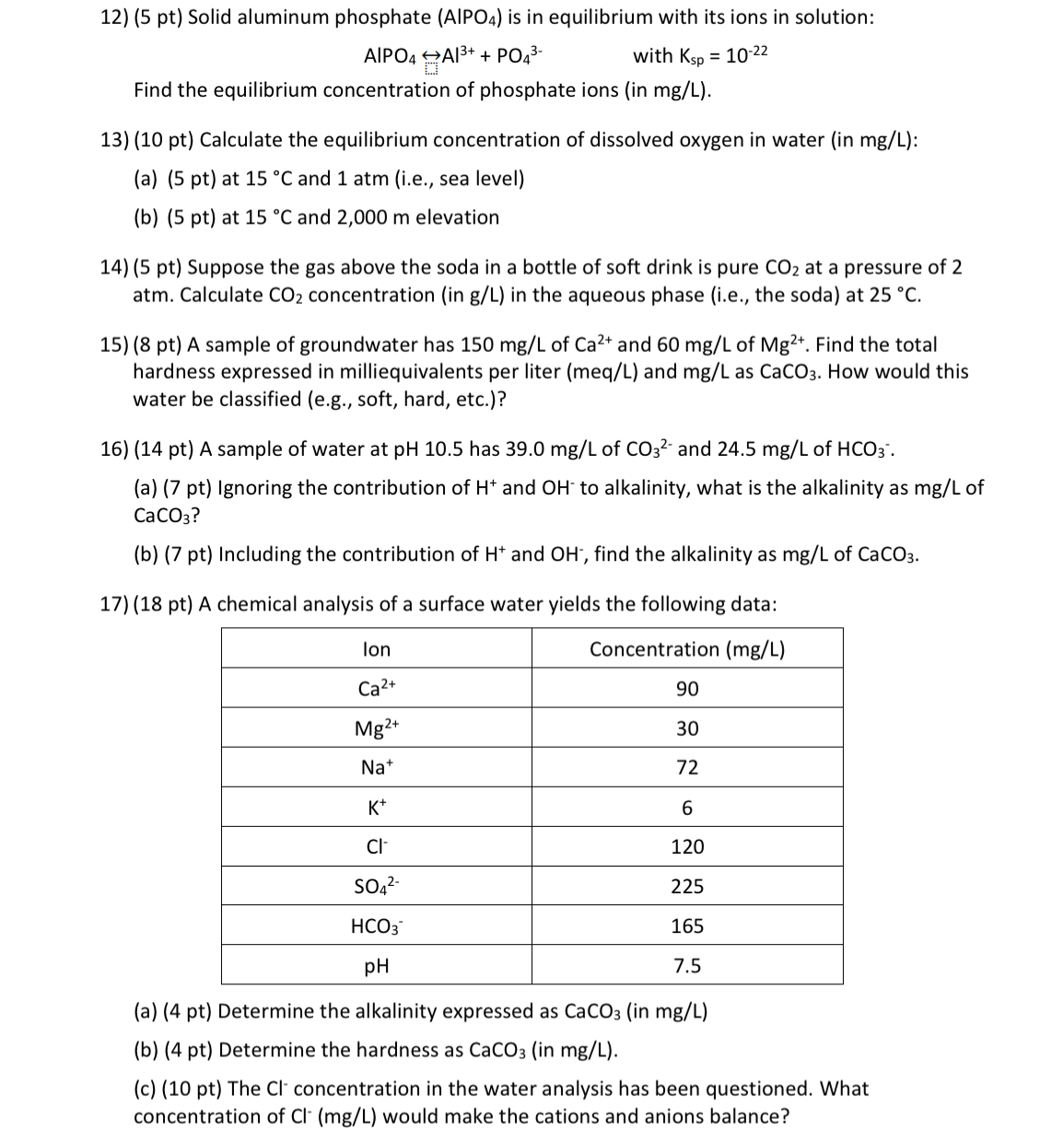
pt A chemical analysis of a surface water yields the following data:
tableIonConcentration mgL
a pt Determine the alkalinity expressed as in
b pt Determine the hardness as in
c pt The concentration in the water analysis has been questioned. What concentration of would make the cations and anions balance?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


