Question: 5. Tabulate the wavelength required to just start a current ow for the 6 available surfaces. Sodium 491 Platinum 185 Zinc 270 Calcium 397 Copper
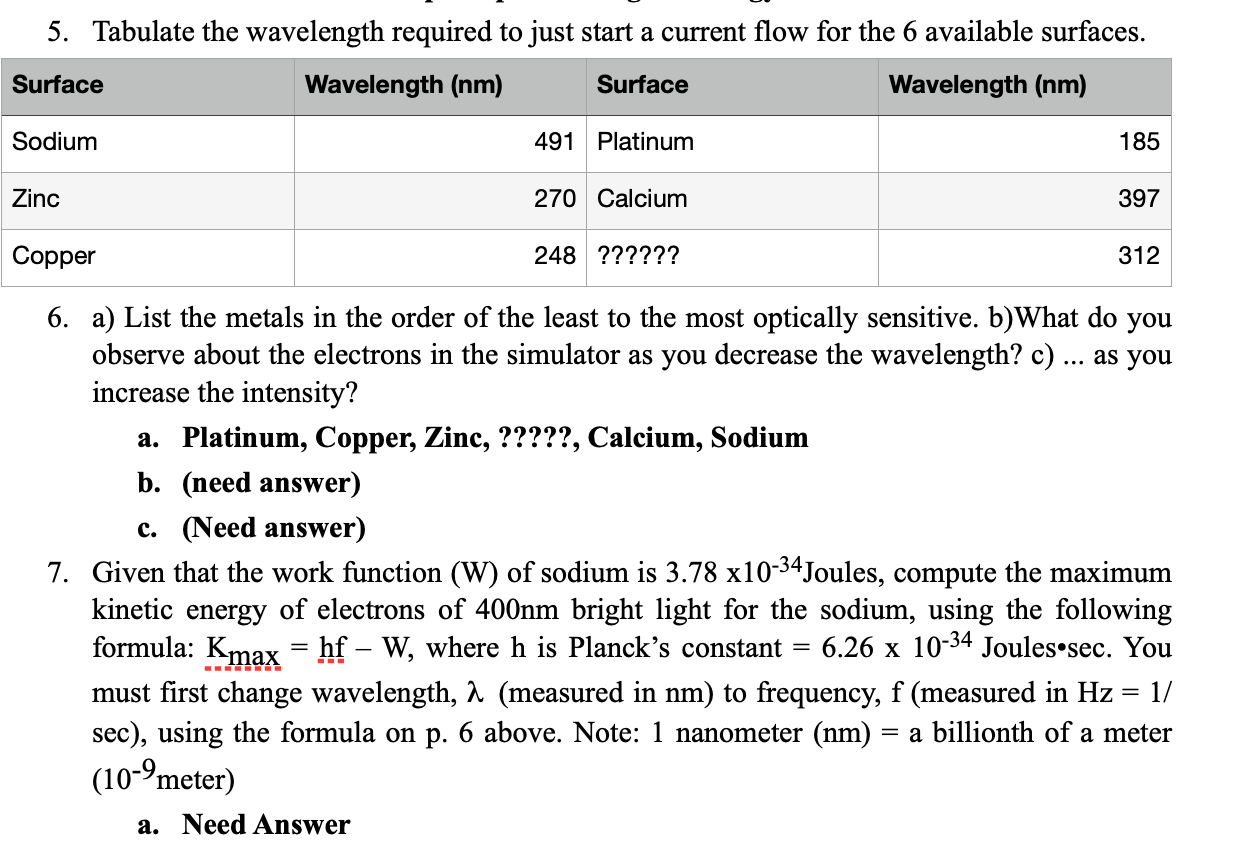
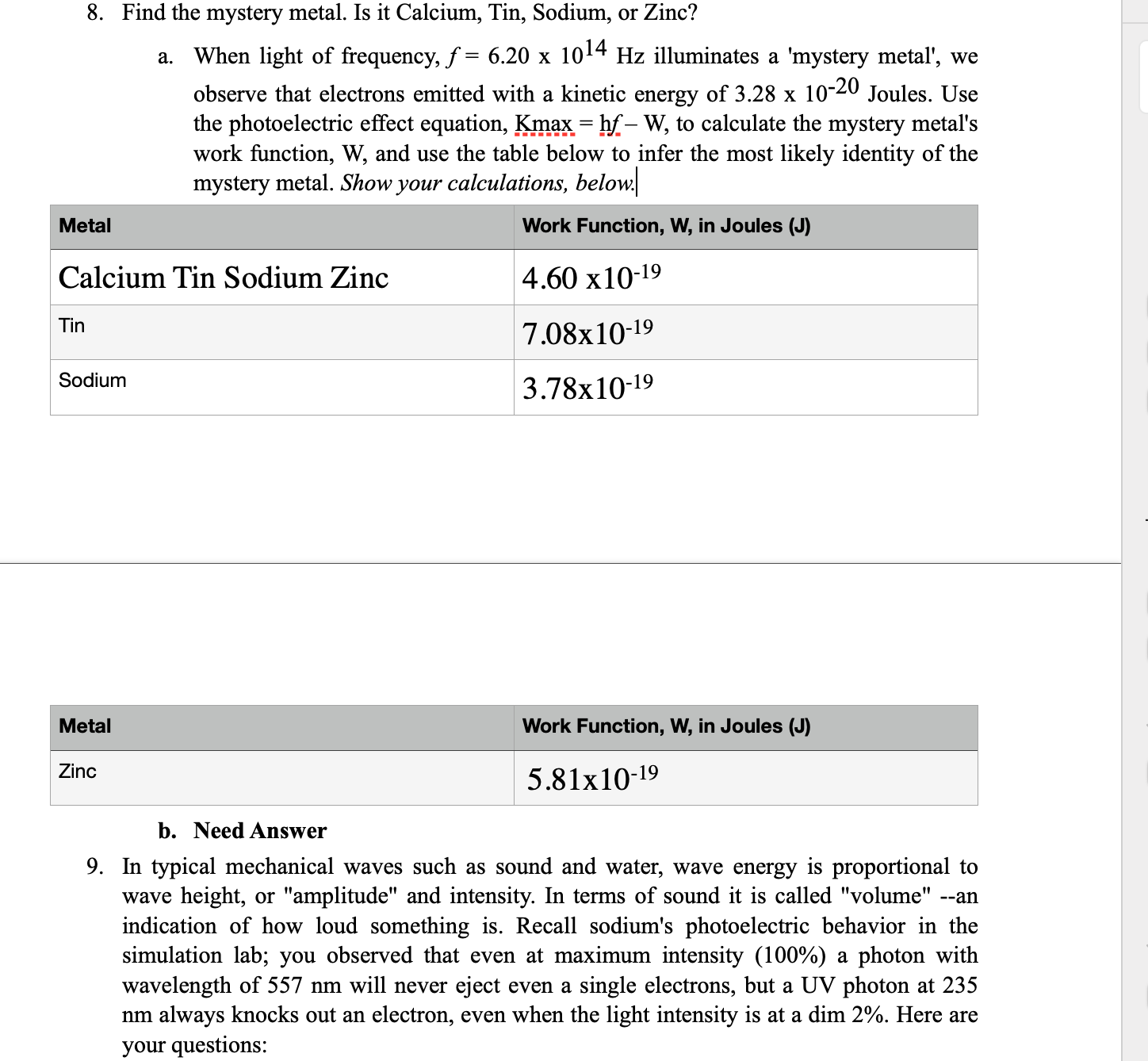

5. Tabulate the wavelength required to just start a current ow for the 6 available surfaces. Sodium 491 Platinum 185 Zinc 270 Calcium 397 Copper 248 ?????? 312 6. a) List the metals in the order of the least to the most optically sensitive. b)What do you observe about the electrons in the simulator as you decrease the wavelength? c) as you increase the intensity? a. Platinum, Copper, Zinc, ?????, Calcium, Sodium b. (need answer) c. (Need answer) 7. Given that the work function (W) of sodium is 3.78 x10'34Joules, compute the maximum kinetic energy of electrons of 400nm bright light for the sodium, using the following formula: Kw = hf W, where h is Planck's constant = 6.26 x 10'34 JouleSsec. You must rst change wavelength, A (measured in nm) to frequency, f (measured in Hz = 1/ sec), using the formula on p. 6 above. Note: 1 nanometer (nm) = a billionth of a meter (1 0'9meter) :1. Need Answer 8. Find the mystery metal. Is it Calcium, Tin, Sodium, or Zinc? a. When light of frequency, f = 6.20 x 1014 Hz illuminates a 'mystery metal', we observe that electrons emitted with a kinetic energy of 3.28 x 10'20 Joules. Use the photoelectric effect equation, Kmax= hf W, to calculate the mystery metal's work Jnction, W, and use the table below to infer the most likely identity of the mystery metal. Show your calculations, belowl Calcium Tin Sodium Zinc 4.60 x10'19 \"" 7.08::10-19 S\"'\"'\" 3.78x10-19 2m\" 5.81;:10-19 b. Need Answer 9. In typical mechanical waves such as sound and water, wave energy is proportional to wave height, or "amplitude" and intensity. In terms of sound it is called "volume" --an indication of how loud something is. Recall sodium's photoelectric behavior in the simulation lab; you observed that even at maximum intensity (100%) a photon with wavelength of 557 nm will never eject even a single electrons, but a UV photon at 235 nm always knocks out an electron, even when the light intensity is at a dim 2%. Here are your questions: your questions: a. Why does this result clearly indicate particle-like properties of light (which Einstein referred to photons) rather than wave-like properties? i. Need Answer b. Use the graph below to describe how Einstein explained the hitherto unexplained example of light's interaction with metal surfaces in photoelectric effect experiments. In particular, i. Need Answer c. What does the graph say about how the color of light related to light's energy (E = hf) i. Need Answer d. how do the absorption spectra of different elements reveal their atomic structure (see pages p. 5 & 6 above. Hint: "atomic structure" = "the way that electrons are distributed around the atomic nucleus." i. Need
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


