Question: 5. The following oxidation occurs over a solid catalyst: If acetone cannot adsorb and the rate of surface reaction between adsorbed isopropanol and adsorbed oxygen
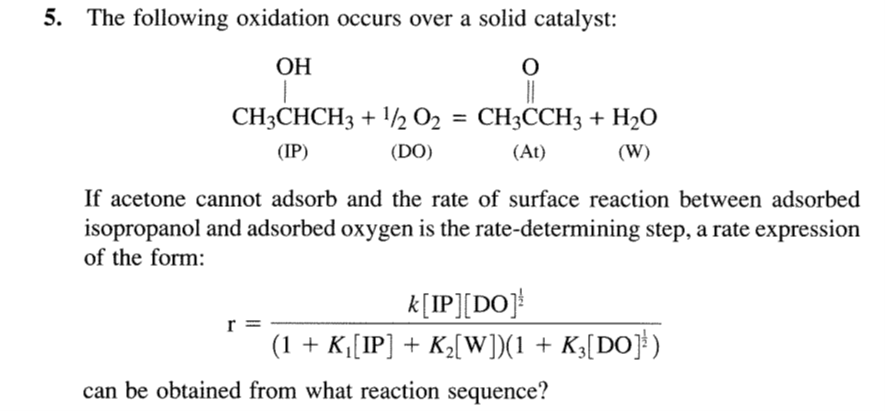
5. The following oxidation occurs over a solid catalyst: If acetone cannot adsorb and the rate of surface reaction between adsorbed isopropanol and adsorbed oxygen is the rate-determining step, a rate expression of the form: r=(1+K1[IP]+K2[W])(1+K3[DO]21)k[IP][DO]21 can be obtained from what reaction sequence
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


