Question: 5. The fugacity for component a in a binary mixture of species a and b is given in the

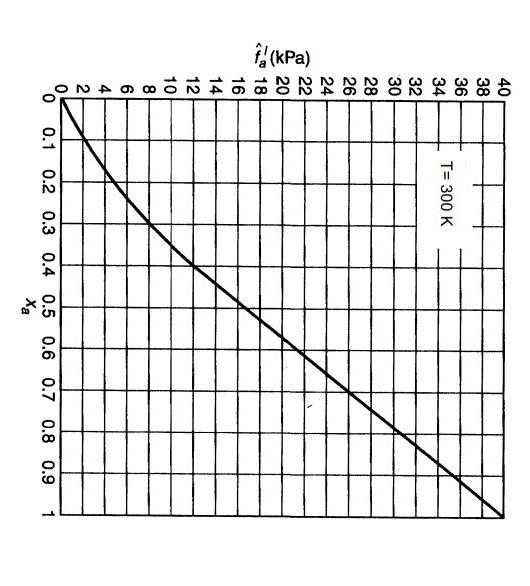
5. The fugacity for component " a " in a binary mixture of species " a " and " b " is given in the figure below. At 300K, the saturation vapor pressure of component b is 28kPa. a) Determine the saturation vapor pressure of component "a" at 300K. b) Determine the Henry's law constant in units of bar for component "a". c) Determine the Henry's law activity coefficient (aHL) for component "a" at x=0.40. d) Determine the Raoult's law activity coefficient for component "a" at x2=0.40. e) The temperature dependence for the Henry's law constant for component "a" is given by: (1/Tlnka)P,nt=540 for k( bar )&T(K). Determine the Henry's law constant at 310 K. State any assumptions made
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


