Question: 5. Using the thermodynamic tables in appendix A3.2 calculate the change in enthalpy when 10.0 g of Cu reacts according to the above equation, assuming

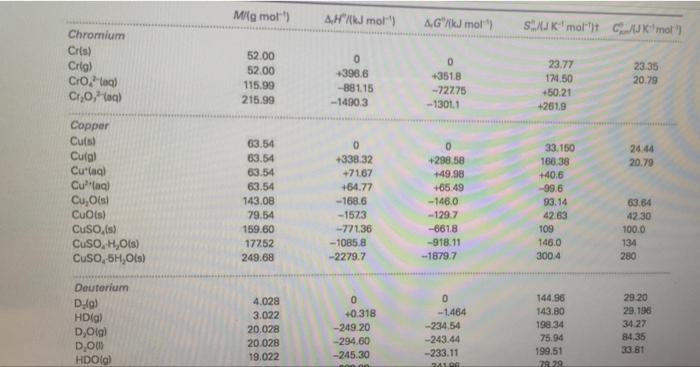


5. Using the thermodynamic tables in appendix A3.2 calculate the change in enthalpy when 10.0 g of Cu reacts according to the above equation, assuming all of the reactants and products are in their standard state. Mig mo) Hmol AG k mol SJUK' molt c'mol Chromium Cris) Crio) Crotal Croa) 52.00 52.00 115.99 215.99 0 +398.6 -881.15 -14903 0 +351.8 -72275 - 13011 23.77 174,50 +50.21 +261.9 23.35 20.79 24.44 20.79 Copper Cus) Culgi Cu(aq) Culag) Cu, Ots Cuis) CuSO,(s) CuSO,H,O(s) CuSO,5H, Ols) 63.54 63.54 63.54 63,54 143.08 79.54 159.60 17252 249.68 0 +338.32 +7167 +84.77 -168,6 -1573 -77136 -1085.8 -2279.7 0 +298,58 149.98 +65.49 -146.0 - 129.7 -861.8 -918.11 -1879.7 33.150 186 38 +40.6 -99.6 93.14 42.63 109 146.0 300.4 63.64 4230 100.0 134 280 0 Deuterium D.4) HD(g) D, Olg) DOUS HDO(g) 4.028 3.022 20.028 20.028 19.022 0 +0.318 -249.20 -294.60 -245.30 0 --1464 -234.54 -243.44 -233.11 144.96 143.80 198.34 75.94 199,51 79229 29.20 29.196 34.27 8435 33 81 4. Copper metal reacts with concentrated nitric acid as shown in this chemical equation: Cu(s) + 4HNO,(aq) C++ (aq) + 2 NO, (aq) + 2 NO2(g) + 2 H20(1) Estimate the amount of pressure-volume work involved when 10.0 g of Cu reacts in a system open to air at a pressure of 760 torr and temperature of 25C. Neglect the volume of the solid and liquids in this reaction. modynamic tables in appendix A3.2 calculate the change in enthalpy Il af the reactants = 0.3148 mol As per the concept of molar volume, 1 mol of a gas occupies 22.4 L at STP (standard conditions of temperature and pressure, P = 1 atm = 760 torr and T = 298 K or 25C). Since the NO2 gas is produced at 760 torr and 25*C, hence, the volume occupied by 0.3148 mol NO2 = (0.3148 mol)" (22.4 L)/(1 mol) = 7.051 L. Given the pressure of the gas is 760 torr = 1 atm, the amount of pressure-volume work done by the reaction = P(change in volume) = P(volume of gas produced) [the reactants are solids and liquids while only NO2 is the gaseous product and the volume change is due to the production of NO2 gas only! (1 atm)'(7.051 L) = 7.051 L-atm 7.05 L-atm (ans, correct to 3 sig, figs)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


