Question: 5.3 Use standard potential data from Resource section 3 as a guide to write balanced equations for the reactions that each of the following species

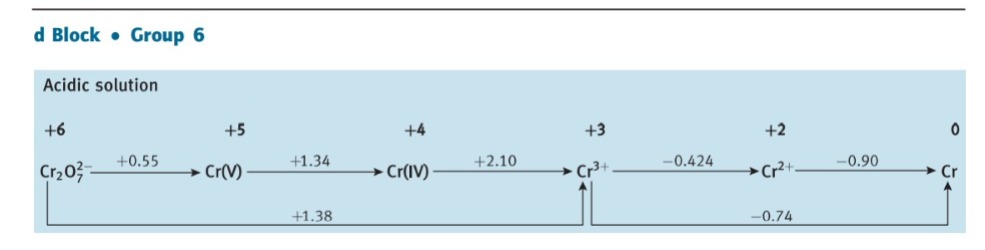
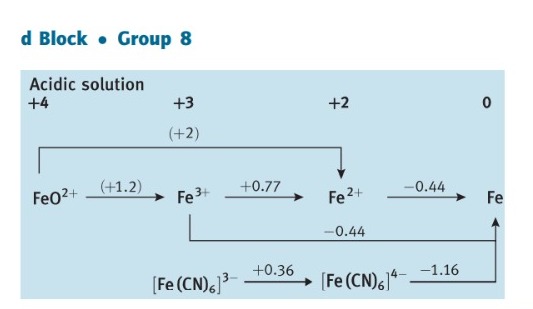
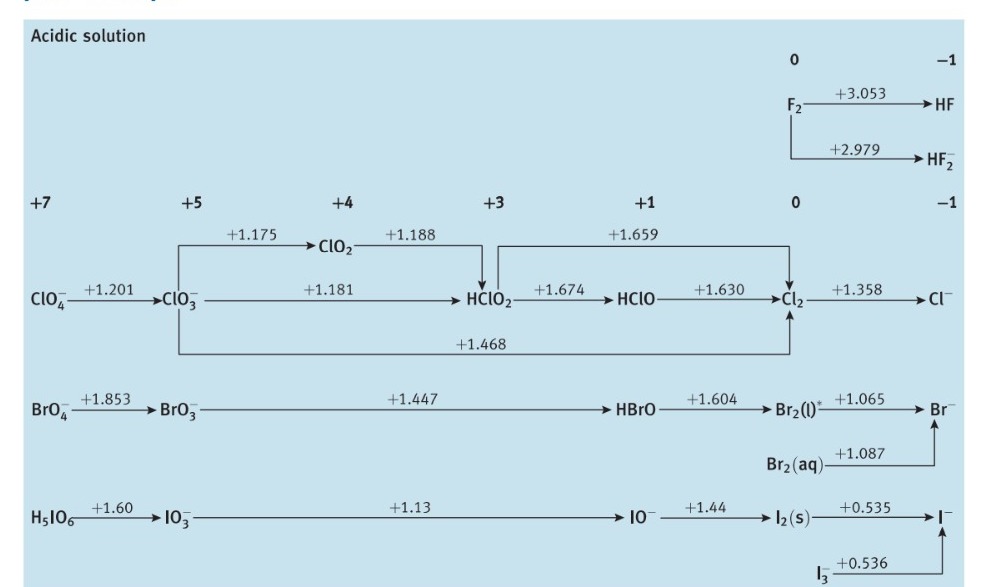
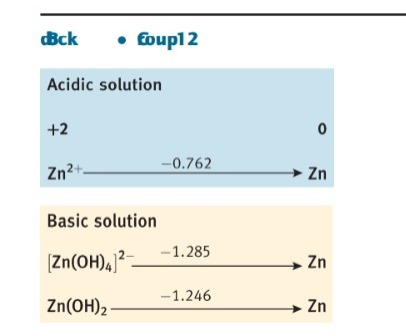
5.3 Use standard potential data from Resource section 3 as a guide to write balanced equations for the reactions that each of the following species might undergo in aerated aqueous acid. If the species is stable, write 'no reaction'. (a) Cr2+, (b) Fe2+, (c) Cl, (d) HClO, (e) Zn(s). d Block Group 6 Acidic solution d Block Group 8 Acidic solution dick - oup12 Acidic solution +2Zn2+0Zn Basic solution [Zn(OH)4]21.285Zn(OH)2Zn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


