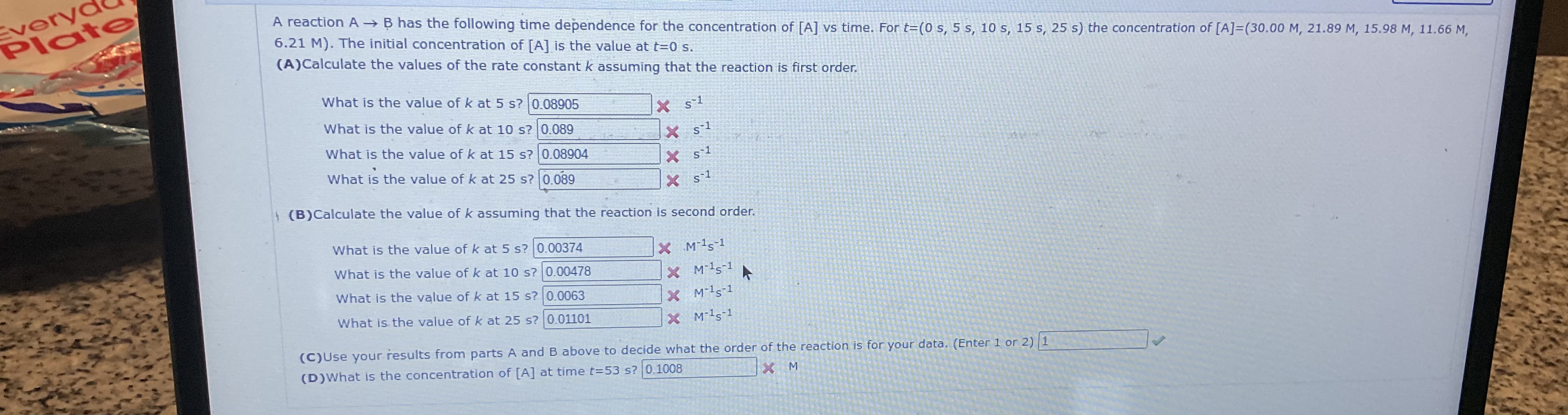
Question: 6 . 2 1 M ) . The initial concentration of A is the value at t = 0 s . ( A ) Calculate
The initial concentration of is the value at
ACalculate the values of the rate constant assuming that the reaction is first order.
What is the value of at
What is the value of at
What is the value of at
What is the value of at
B Calculate the value of assuming that the reaction is second order.
C Use your results from parts A and above to decide what the order of the reaction is for your data. Enter or
D What is the concentration of at time
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


