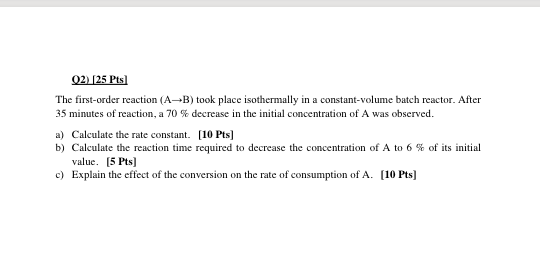
Question: please answer clearly and quickly 02) [25 Pts) The first-order reaction ( AB) took place isothermally in a constant-volume batch reactor. After 35 minutes of
 please answer clearly and quickly
please answer clearly and quickly
02) [25 Pts) The first-order reaction ( AB) took place isothermally in a constant-volume batch reactor. After 35 minutes of reaction, a 70 % decrease in the initial concentration of A was observed. a) Calculate the rate constant. [10 Pts) b) Calculate the reaction time required to decrease the concentration of A to 6 % of its initial value. (5 Pts) c) Explain the effect of the conversion on the rate of consumption of A. [10 Pts]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


