Question: 6) Consider two reactors in series and the molar flow rate is 68 mol/min. If the conversion of first reactor is 30% and the conversion
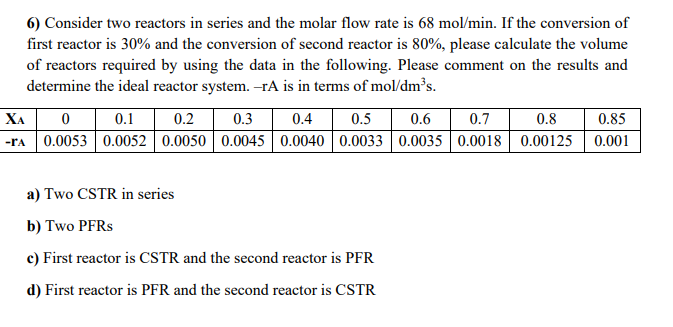
6) Consider two reactors in series and the molar flow rate is 68 mol/min. If the conversion of first reactor is 30% and the conversion of second reactor is 80%, please calculate the volume of reactors required by using the data in the following. Please comment on the results and determine the ideal reactor system. TA is in terms of mol/dms. XA0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85 -DA 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0035 0.0018 0.00125 0.001 a) Two CSTR in series b) Two PFRs c) First reactor is CSTR and the second reactor is PFR d) First reactor is PFR and the second reactor is CSTR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


